- Record: found
- Abstract: found
- Article: not found
Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial
Read this article at
Abstract
Background
The oral, selective Janus kinase 1/2 inhibitor baricitinib has shown efficacy in studies of hospitalised adults with COVID-19. COV-BARRIER (NCT04421027) was a multinational, phase 3, randomised, double-blind, placebo-controlled trial of baricitinib in patients with confirmed SARS-CoV-2 infection. We aimed to evaluate the efficacy and safety of baricitinib plus standard of care in critically ill hospitalised adults with COVID-19 requiring invasive mechanical ventilation or extracorporeal membrane oxygenation.
Methods
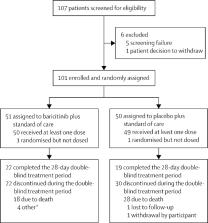
This exploratory trial followed the study design of COV-BARRIER in a critically ill cohort not included in the main phase 3 trial. The study was conducted across 18 hospitals in Argentina, Brazil, Mexico, and the USA. Participants (aged ≥18 years) hospitalised with laboratory-confirmed SARS-CoV-2 infection on baseline invasive mechanical ventilation or extracorporeal membrane oxygenation were randomly assigned (1:1) to baricitinib (4 mg) or placebo once daily for up to 14 days in combination with standard of care. Participants, study staff, and investigators were masked to study group assignment. Prespecified endpoints included all-cause mortality through days 28 and 60, number of ventilator-free days, duration of hospitalisation, and time to recovery through day 28. The efficacy analysis was done in the intention-to-treat population and the safety analysis was done in the safety population. This trial is registered with ClinicalTrials.gov, NCT04421027.
Findings
Between Dec 23, 2020, and April 10, 2021, 101 participants were enrolled into the exploratory trial and assigned to baricitinib (n=51) or placebo (n=50) plus standard of care. Standard of care included baseline systemic corticosteroid use in 87 (86%) participants. Treatment with baricitinib significantly reduced 28-day all-cause mortality compared with placebo (20 [39%] of 51 participants died in the baricitinib group vs 29 [58%] of 50 in the placebo group; hazard ratio [HR] 0·54 [95% CI 0·31–0·96]; p=0·030; 46% relative reduction; absolute risk reduction 19%). A significant reduction in 60-day mortality was also observed in the baricitinib group compared with the placebo group (23 [45%] events vs 31 [62%]; HR 0·56 [95% CI 0·33–0·97]; p=0·027; 44% relative reduction; absolute risk reduction 17%). In every six baricitinib-treated participants, one additional death was prevented compared with placebo at days 28 and 60. The number of ventilator-free days did not differ significantly between treatment groups (mean 8·1 days [SD 10·2] in the baricitinib group vs 5·5 days [8·4] in the placebo group; p=0·21). The mean duration of hospitalisation in baricitinib-treated participants was not significantly shorter than in placebo-treated participants (23·7 days [SD 7·1] vs 26·1 days [3·9]; p=0·050). The rates of infections, blood clots, and adverse cardiovascular events were similar between treatment groups.
Interpretation
In critically ill hospitalised patients with COVID-19 who were receiving invasive mechanical ventilation or extracorporeal membrane oxygenation, treatment with baricitinib compared with placebo (in combination with standard of care, including corticosteroids) reduced mortality, which is consistent with the mortality reduction observed in less severely ill patients in the hospitalised primary COV-BARRIER study population. However, this was an exploratory trial with a relatively small sample size; therefore, further phase 3 trials are needed to confirm these findings.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: not found
