- Record: found
- Abstract: found
- Article: found
Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes
Read this article at
Abstract
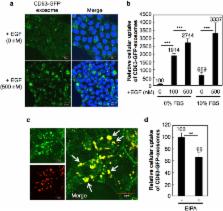
Exosomes are approximately 100-nm vesicles that consist of a lipid bilayer of cellular membranes secreted in large quantities from various types of normal and disease-related cells. Endocytosis has been reported as a major pathway for the cellular uptake of exosomes; however, the detailed mechanisms of their cellular uptake are still unknown. Here, we demonstrate the active induction of macropinocytosis (accompanied by actin reorganisation, ruffling of plasma membrane, and engulfment of large volumes of extracellular fluid) by stimulation of cancer-related receptors and show that the epidermal growth factor (EGF) receptor significantly enhances the cellular uptake of exosomes. We also demonstrate that oncogenic K-Ras-expressing MIA PaCa-2 cells exhibit intensive macropinocytosis that actively transports extracellular exosomes into the cells compared with wild-type K-Ras-expressing BxPC-3 cells. Furthermore, encapsulation of the ribosome-inactivating protein saporin with EGF in exosomes using our simple electroporation method produces superior cytotoxicity via the enhanced cellular uptake of exosomes. Our findings contribute to the biological, pharmaceutical, and medical research fields in terms of understanding the macropinocytosis-mediated cellular uptake of exosomes with applications for exosomal delivery systems.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Regulated portals of entry into the cell.
- Record: found
- Abstract: found
- Article: not found
Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis.

- Record: found
- Abstract: found
- Article: found