- Record: found
- Abstract: found
- Article: found
Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography
Read this article at
Abstract
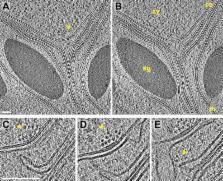
Chloroplast function is orchestrated by the organelle's intricate architecture. By combining cryo-focused ion beam milling of vitreous Chlamydomonas cells with cryo-electron tomography, we acquired three-dimensional structures of the chloroplast in its native state within the cell. Chloroplast envelope inner membrane invaginations were frequently found in close association with thylakoid tips, and the tips of multiple thylakoid stacks converged at dynamic sites on the chloroplast envelope, implicating lipid transport in thylakoid biogenesis. Subtomogram averaging and nearest neighbor analysis revealed that RuBisCO complexes were hexagonally packed within the pyrenoid, with ∼15 nm between their centers. Thylakoid stacks and the pyrenoid were connected by cylindrical pyrenoid tubules, physically bridging the sites of light-dependent photosynthesis and light-independent carbon fixation. Multiple parallel minitubules were bundled within each pyrenoid tubule, possibly serving as conduits for the targeted one-dimensional diffusion of small molecules such as ATP and sugars between the chloroplast stroma and the pyrenoid matrix.
eLife digest
Many organisms can harvest light to produce their own energy through a process called photosynthesis. In plant and algal cells, photosynthesis takes place within the chloroplasts, which are compartments that contain stacks of structures called thylakoids.
Inside the thylakoids, proteins absorb energy from light and convert it into biochemical energy that can be used by the cell. This energy then powers a series of reactions that result in carbon dioxide being incorporated into energy-rich sugars. The enzyme RuBisCO is essential for this process, and is believed to be the most abundant protein on Earth. In land plants, RuBisCO is found throughout the chloroplast, but in algae it is limited to a specialized area called the pyrenoid.
Much of our current knowledge of chloroplast structure comes from transmission electron microscopy (TEM) images. However, the traditional methods used to prepare cells for TEM can damage their internal structures. Also, previous studies have focused primarily on the chloroplasts of land plants, even though aquatic organisms—including the alga Chlamydomonas—account for over 50% of photosynthesis on the planet.
Here, Engel et al. provide the first three-dimensional structures of Chlamydomonas chloroplasts in their natural state. They used several recently-developed techniques to study cells that were preserved in a close-to-living condition. The cells were rapidly frozen, thinned with a technique called cryo-focused ion beam milling, and then imaged by a type of TEM called cryo-electron tomography.
The three-dimensional images provide many insights into the Chlamydomonas chloroplast, including evidence that lipids and proteins move between the membrane that surrounds the chloroplast—called the chloroplast envelope—and the tips of the thylakoids. These images show how thylakoids may be built by the transport of molecules from the chloroplast envelope. In addition, the images reveal the detailed structures of the tubes that connect the thylakoids to the pyrenoid, which could explain how the two stages of photosynthesis (light harvesting and the conversion of carbon dioxide) can be coordinated even though they occur at different places within the chloroplast.
Engel et al. also observed that RuBisCO enzymes are arranged in a hexagonal pattern inside the pyrenoid, but are spaced too far apart to make direct contact with each other. To understand how the pyrenoid is assembled, a future goal will be to determine what causes RuBisCO to be arranged in this way.
Related collections
Most cited references104
- Record: found
- Abstract: not found
- Article: not found
Multidimensional binary search trees used for associative searching
- Record: found
- Abstract: not found
- Article: not found
Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components
- Record: found
- Abstract: found
- Article: not found