INTRODUCTION
With the demand for donor lungs continuously outpacing their availability, transplant surgeons have long sought temporizing measures to sustain recipients awaiting transplantation. Although mechanical ventilation has been used as a conventional bridging modality, certain pathologic aspects of candidates awaiting lung transplantation limit the practical utility of this option (e.g., idiopathic pulmonary fibrosis and primary pulmonary hypertension) including chronic airway damage resulting from barotrauma associated with prolonged positive pressure ventilation. Heretofore, extracorporeal membrane oxygenation (ECMO) has been used infrequently as an alternative bridge to lung transplantation with mixed outcomes [1–2]. Within the past few years, however, there has been renewed interest in using this bridging technology in waiting recipients who otherwise would not have survived. Advantages of ECMO over mechanical ventilation as a bridging modality lie in its capacity to not only rest the lung parenchyma and avoid the complications associated with prolonged intubation but also to reliably achieve full oxygenation and adequate carbon dioxide removal.
Prior single institution studies have been encouraging but handicapped with small patient cohorts [1–6]. We present data from the United Network of Organ Sharing (UNOS) database on the outcomes of patient bridged to lung transplantation using ECMO to reflect the global experience among centers applying this bridging strategy. This data can be used to help refine application of ECMO to this challenging patient population.
MATERIAL AND METHODS
The Yale University School of Medicine Institutional Review Board approved this study. A retrospective analysis of the UNOS database, from January 2000 to December 2011, was performed to compare the outcomes of lung and heart–lung transplant recipients that were bridged to transplant with ECMO versus those recipients that were not (control group). During these 11 years, 14,263 lung transplants were performed, of which, 143 patients (1.0%) were bridged to transplant using ECMO. Both primary and retransplants were included. Only patients 18 years old or older were included in the study. As the database does not separate veno-arterial from veno-venous ECMO, all bridged patients were included regardless of the type of ECMO. Donor and recipient characteristics were compared for each group with univariate and multivariate analysis using SAS 9.3 (Cary, NC, USA) statistical software.
The primary end points included 30-day, 3-month, 6-month, 1-year, 2-year, 3-year, 5-year, and 10-year mortalities. Continuous variables are presented as a mean ± SD and the range. Categorical variables are displayed as frequency of distribution (n) and percentage (%). Kaplan–Meier curves were used to show survival outcomes. The p-values for continuous variables were calculated using a Student's t-test, and chi-square analysis was used for categorical variables. For all comparisons, two-tailed p-values less than 0.05 were considered statistically significant. Multivariate analysis was performed using the Cox proportional hazards model. All variables that showed statistically significant results using univariate analysis were included in the multivariate analysis.
RESULTS
Donor characteristics
Donor characteristics were similar between the two groups (Table 1) with the exception that there was a higher percentage of lungs from male donors that were transplanted into the control group (60.0% vs. 51.1%, p = 0.03). Multivariate analysis did not show this to be statistically significant (p = 0.389, HR: 0.96, 95% CI: 0.875–1.05). The causes of death among donors was not statistically significant between the two groups (p = 0.07) with the most common causes for both groups being head injury and stroke accounting for 79.72% of lungs used in the ECMO group, and 87.3% in the control group (Table 1). There were a higher percentage of lungs used from patients that died from anoxia in the ECMO group (16.8% vs. 9.6%). The causative mechanisms of death among the donors were also similar between the two groups, with the most common mechanisms being gunshot wounds, blunt injury, and stroke. Notably, 3.5% of donor lungs in the ECMO group and 2.0% of donor lungs in the control group were taken from people who died of asphyxiation.
| ECMO group | No ECMO group | p-value | |||
|---|---|---|---|---|---|
| Average (n = 143) ± SD | Range | Average (n = 14120) ± SD | Range | ||
| Age (years) | 34.3 ± 14.3 | 10–73 | 33.1 ± 14.1 | 2–76 | 0.324 |
| Gender | |||||
| Male | 73 (51.05%) | 8471 (60.0%) | 0.0299 | ||
| Female | 70 (48.95%) | 5649 (40.0%) | |||
| BMI | 25.5 ± 5.3 | 18.1–43.6 | 25.1 ± 4.9 (14107) | 10.8–54.9 | 0.2754 |
| pO2 | 397.5 ± 153.4 | 47.0–619 | 402.2 ± 142.2 | 30.7–754.0 | 0.693 |
| Donor type | |||||
| Deceased | 143 (100%) | 14060 (99.6%) | 0.4347 | ||
| History of cigarette use | 25 (17.48%) | 2486 (17.67%) | 0.998 | ||
| Donor pulmonary infection | 52 (36.4%) | 4249 (30.2%) | 0.1117 | ||
| Donor inotropic medications at procurement | 81 (57.5%) | 8533 (63.1%) | 0.3706 | ||
| Donor cause of death | n = 143 | n = 14058 | 0.0668 | ||
| Anoxia | 24 (16.78%) | 1347 (9.58%) | |||
| Cerebrovascular/stroke | 47 (32.87%) | 5061 (36.0%) | |||
| Head trauma | 67 (46.85%) | 7208 (51.27%) | |||
| CNS tumor | 1 (0.7%) | 126 (0.9%) | |||
| Other | 4 (2.8%) | 316 (2.25%) | |||
| Donor mechanism of death | n = 143 | n = 14086 | 0.0179 | ||
| Drowning | 0 (0%) | 29 (0.2%) | |||
| Seizure | 2 (1.4%) | 123 (0.9%) | |||
| Drug intoxication | 7 (4.9%) | 353 (2.5%) | |||
| Asphyxiation | 5 (3.5%) | 276 (2.0%) | |||
| Cardiovascular | 13 (9.1%) | 651 (4.4%) | |||
| Gunshot wound | 17 (11.9%) | 2869 (20.4%) | |||
| Stab | 0 (0%) | 21 (0.2%) | |||
| Blunt injury | 45 (31.5%) | 4004 (28.5%) | |||
| Intracranial hemorrhage/stroke | 48 (33.6%) | 5306 (37.7%) | |||
| Death from natural causes | 0 (0%) | 146 (1.0%) | |||
| None of the above | 6 (4.2%) | 308 (2.2%) | |||
Recipient characteristics
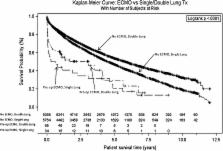
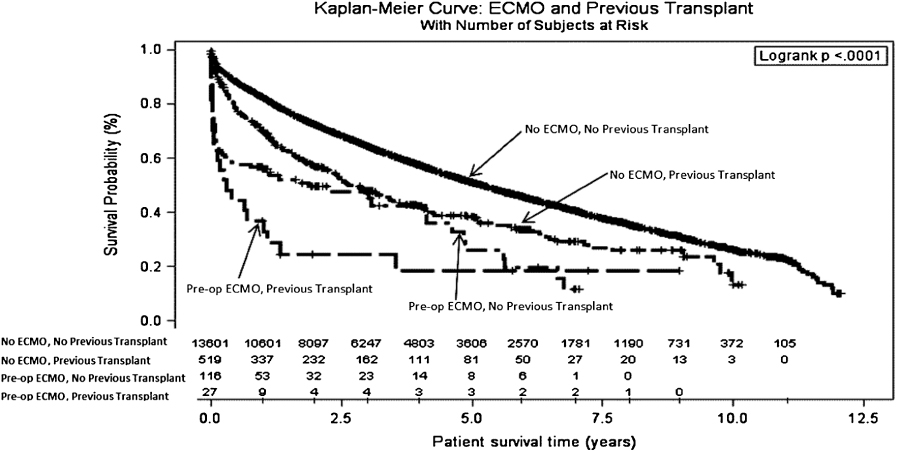
The recipients in the ECMO group tended to be younger, with an average age of 47 years versus 52 years in the control group (p ≤ 0.01) (Table 2). The control group recipients were more likely to have a history of tobacco use (62% versus 47%, p = 0.01). This, however, was not shown to be significant using multivariable analysis. ECMO recipients also were more likely to require pretransplant dialysis 12% versus 0.4% (p ≤ 0.01), more likely to have a history of malignancy 7% versus 5% (p ≤ 0.01), more likely to have had prior lung surgery (nontransplant) between the time they were listed and the time they were transplanted (21% versus 8%, p ≤ 0.01, and more likely to have had a previous lung transplant (19% versus 4%, p ≤ 0.01). None of these differences, however, were found to be statistically significant by multivariate analysis (Table 3).
| ECMO group | No ECMO group | p-value | |||
|---|---|---|---|---|---|
| Average (n = 143) ± SD | Range | Average (n = 14120) ± SD | Range | ||
| Age (years) | 47.3 ± 15.0 | 18–74 | 52.2 ± 13.0 | 18–81 | <0.0001 |
| Gender | |||||
| Male | 80 (55.9%) | 7783 (55.1%) | 0.8438 | ||
| Female | 63 (44.1%) | 6337 (44.9%) | |||
| BMI Recipient | 25.0 ± 5.3 | 13.7–37.2 | 24.5 ± 4.8 | 7.3–54.0 | 0.2154 |
| Diabetes | 29 (20.3%) | 2107 (14.9%) | 0.074 | ||
| History of cigarette use | 51 (46.4%) | 5331 (62.0%) | 0.0008 | ||
| Chronic steroid use | 76 (53.2%) | 6688 (47.4%) | 0.0575 | ||
| Pretransplant dialysis since listing | 17 (11.9%) | 56 (0.4%) | <0.0001 | ||
| History of malignancy | 10 (7.0%) | 682 (4.8%) | <0.0001 | ||
| Lung surgery Between listing and transplant (nontransplant) | 30 (21.0%) | 1105 (7.8%) | <0.0001 | ||
| Prior lung surgery | 8 (15.7%) | 1318 (16.6%) | 0.4313 | ||
| Previous transplants | 27 (18.9%) | 519 (3.7%) | <0.0001 | ||
| Days between previous and current transplant | 516.1 ± 1166.2 | 1.0–4729 | 1518.5 ± 1299.5 | 1.0–6898 | <0.0001 |
| Days waiting from initial date to end date | 170.4 ± 336.9 | 0–2151 | 319.7 ± 447.7 | 0–5843 | <0.0001 |
| PA mean pressures (mmHg) | 32.1 ± 14.7 | 4.0–84.0 | 27.6 ± 11.8 | 0.0–110.0 | 0.0002 |
| Pulmonary capillary wedge pressures (mmHg) | 11.7 ± 7.9 | 1.0–39.0 | 11.2 ± 5.9 | 0.0–50.0 | 0.4143 |
| Cardiac output (L/min) | 5.3 ± 1.8 | 2.0–15.0 | 5.3 ± 1.5 | 0.2–15.0 | 0.8576 |
| O2 requirements (L/min) | 8.0 ± 7.0 | 0–20 | 2.9 ± 2.4 | 0–20 | <0.0001 |
| pCO2 (mmHg) | 47.2 ± 16.8 | 10.0–107.0 | 46.6 ± 12.7 | 10.0–120.0 | 0.7105 |
| FEV1 (%) | 42.5 ± 22.0 | 11.0–105.0 | 36.5 ± 21.0 | 5.0–120.0 | 0.0059 |
| FVC (%) | 47.8 ± 19.8 | 11.0–98.0 | 49.7 ± 17.7 | 10.0–130.0 | 0.3079 |
| Creatinine | 1.08 ± 0.81 | 0.20–7.01 | 0.90 ± 0.52 | 0.10–20.0 | <0.0001 |
| Total bilirubin (mg/dL) | 1.25 ± 2.09 | 0.1–21.4 | 0.72 ± 1.59 | 0.1–82.0 | 0.0001 |
| Episodes of ventilator support | 76 (53.2%) | 801 (5.7%) | <0.0001 | ||
| Life support | 143 (100%) | 973 (6.9%) | <0.0001 | ||
| Ischemia time (Hours) | 5.43 ± 1.87 | 0.2–11.75 | 4.90 ± 1.70 | 0.17–12.62 | 0.0003 |
| Length of hospital stay (transplant to discharge (days) | 41.2 ± 45.4 | 0–261 | 24.5 ± 30.3 | 0–566 | <0.0001 |
| Lung allocation score at match | 68.9 ± 22.9 | 28.4–95.2 | 44.2 ± 15.1 | 0–95.5 | <0.0001 |
| Transplant type | n = 129 | n = 13820 | 0.0004 | ||
| Single lung | 34 (26.4%) | 5764 (41.7%) | |||
| Double lung | 95 (73.6%) | 8056 (58.3%) | |||
| Hazards ratio | 95% confidence interval | Standard error | p-value | |
|---|---|---|---|---|
| Donor gender | 1.072 | 0.968–1.187 | 0.05 | 0.1808 |
| Recipient age | 1.012 | 1.006–1.018 | 0.003 | <0.0001 |
| History of cigarette use | 0.934 | 0.83–1.051 | 0.06 | 0.2591 |
| Pretransplant dialysis—since listing | 1.34 | 0.724–2.478 | 0.31 | 0.3513 |
| Previous malignancy | 1.029 | 0.86–1.231 | 0.09 | 0.756 |
| Previous transplant | 0.986 | 0.677–1.437 | 0.19 | 0.9411 |
| Transplant type (single lung) | 0.957 | 0.853–1.075 | 0.06 | 0.4628 |
| Ischemia time | 1.015 | 0.985–1.045 | 0.02 | 0.3404 |
| Lung allocation score at match | 1.009 | 1.005–1.013 | 0.002 | <0.0001 |
| Lung surgery between listing and transplant (nontransplant) | 0.956 | 0.814–1.123 | 0.08 | 0.5855 |
| Life support | 0.469 | 0.221–0.993 | 0.38 | 0.048 |
| Intraortic balloon pump | 5.368 | 0.583–49.421 | 1.13 | 0.1379 |
| Prostacyclin infusion | 2.218 | 0.832–5.441 | 0.48 | 0.115 |
| Prostacyclin inhalation | 4.067 | 1.30–12.725 | 0.58 | 0.0159 |
| Inhaled nitric oxide | 0.709 | 0.319–1.578 | 0.41 | 0.3994 |
| Ventilator support | 2.698 | 1.262–5.767 | 0.39 | 0.0104 |
| Other life support | 2.81 | 1.357–5.818 | 0.37 | 0.0054 |
| Episodes of ventilator support | 0.903 | 0.724–1.125 | 0.11 | 0.3625 |
| PA mean pressures | 1.005 | 1.000–1.010 | 0.003 | 0.356 |
| FEV1 | 1.003 | 1.000–1.006 | 0.002 | 0.0838 |
| Creatinine | 1.051 | 0.98–1.128 | 0.04 | 0.1632 |
| Total bilirubin | 1.048 | 1.026–1.071 | 0.01 | <0.0001 |
| Length of hospital stay (Transplant to discharge [days]) | 1.002 | 1.001–1.003 | 0 | 0.0002 |
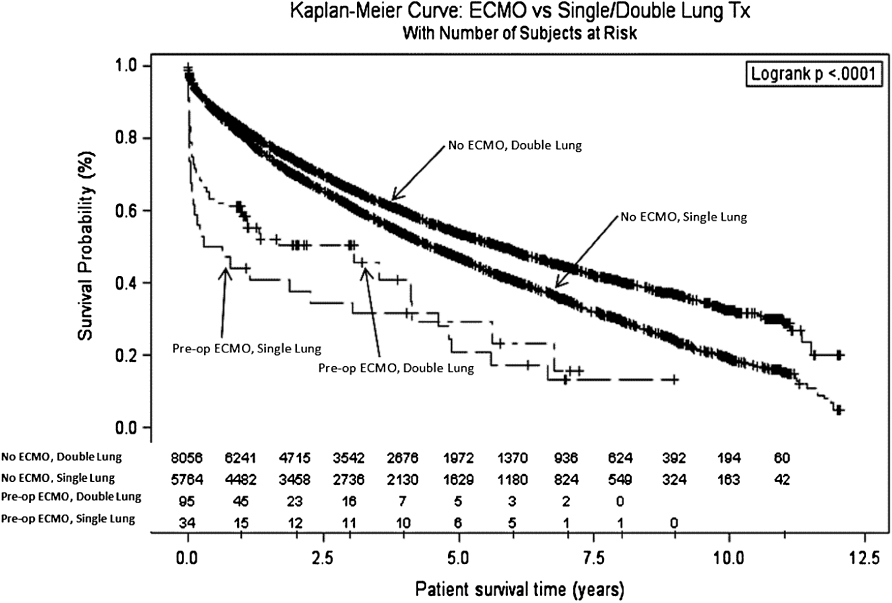
ECMO patients were generally sicker with higher mean pulmonary arterial pressures and supplemental oxygen requirements. They were also more likely to be on some type of life support (i.e., mechanical ventilation, inotropes), and their hospital stays were longer (41 days versus 25 days, p ≤ 0.01). Fifty-one percent of ECMO patients were mechanically ventilated prior to transplant versus just 4% in the control group (p ≤ 0.01). As would be expected, the Lung Allocation Score (LAS) was higher for the ECMO group (p ≤ 0.01). ECMO patients were also more likely to receive a double-lung transplant as opposed to a single-lung transplant (74% versus 58%, p = 0.01), and had greater graft ischemic times (p = 0.01). The most common diagnoses among transplant recipients were Idiopathic Pulmonary Fibrosis (IPF), Chronic Obstructive Lung Disease (COPD)/Emphysema, and Cystic Fibrosis (Table 4). The most notable difference in the preoperative diagnoses was that 9.1% of ECMO patients and only 0.5% of control group patients were retransplanted for acute rejection/primary graft failure.
| ECMO group (n = 122) | No ECMO group (n = 13,194) | p-value | |
|---|---|---|---|
| Diagnosis (most common) | |||
| Scleroderma—restrictive | 2 (1.4%) | 41 (0.3%) | <0.0001 |
| Scleroderma—pulmonary hypertension | 3 (2.1%) | 78 (0.6%) | |
| Hypersensitivity pneumonitis | 2 (1.4%) | 55 (0.4%) | |
| Lupus | 2 (1.4%) | 5 (0.04%) | |
| Lung retransplant/graft failure: obliterative bronchiolitis | 7 (4.9%) | 271 (1.9%) | |
| Lung retransplant/graft failure: acute rejection | 2 (1.4%) | 10 (0.07%) | |
| Lung retransplant/graft failure: primary graft failure | 13 (9.1%) | 71 (0.5%) | |
| Primary pulmonary hypertension | 6 (4.2%) | 394 (2.8%) | |
| Cystic fibrosis | 12 (8.4%) | 1811 (12.8%) | |
| Idiopathic pulmonary Fibrosis/usual interstitial Pneumonitis | 38 (26.6%) | 3786 (26.8%) | |
| Sarcoidosis | 3 (2.1%) | 454 (3.2%) | |
| Alpha-1 antitrypsin deficiency | 2 (1.4%) | 599 (4.2%) | |
| COPD/emphysema | 21 (14.7%) | 4564 (32.3%) | |
| Bronchiectasis | 1 (0.7%) | 251 (1.8%) | |
| Pulmonary fibrosis—other | 6 (4.2%) | 466 (3.3%) | |
| Other | 2 (1.4%) | 338 (2.4%) | |
Survival
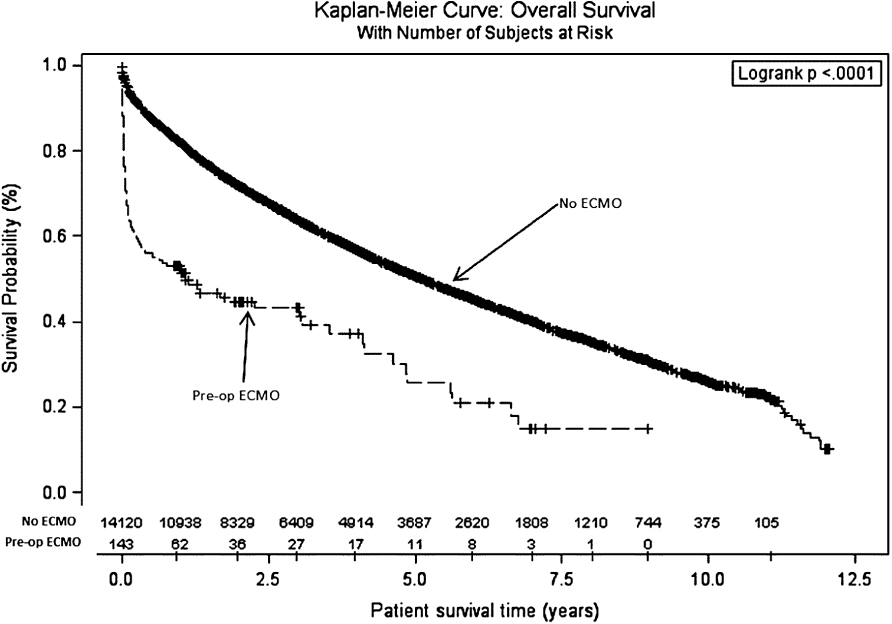
The 30-day, 90-day, 6-month, 1-year, 3-year, 5-year, and 10-year survival rates were 68.5%, 59.4%, 55.9%, 47.7%, 25.5%, 11.2%, and 0%, respectively, for the ECMO group and 95.1%, 91.3%, 87.6%, 81.0%, 57.5%, 38.4%, and 5.1%, respectively, for the control group (p ≤ 0.01) (Table 5). Kaplan–Meier curves for both groups are illustrated in Figure 1. Although the donor and the recipient characteristics were similar, patients bridged with ECMO were, in general, sicker requiring ventilator support while on the list 53.2% of the time versus 5.7% in the control group (p ≤ 0.01) and more frequently required other forms of life support.
| ECMO group (n = 143) | No ECMO group (n = 14117) | p-value | |
|---|---|---|---|
| Patient survival | |||
| 30 days | 98 (68.53%) | 13,419 (95.1%) | <0.0001 |
| 90 days | 85 (59.4%) | 12,883 (91.3%) | <0.0001 |
| 6 months | 80 (55.9%) | 12,339 (87.6%) | <0.0001 |
| 1 year | 62 (47.7%) | 10,938 (81.0%) | <0.0001 |
| 2 years | 36 (31.6%) | 8329 (68.3%) | <0.0001 |
| 3 years | 27 (25.47%) | 6409 (57.5%) | <0.0001 |
| 5 years | 11 (11.2%) | 3687 (38.4%) | <0.0001 |
| 10 years | 0 (0%) | 375 (5.1%) | 0.027 |
When survival outcomes were separated out on the basis of prior transplant, retransplant patients exhibited worse survival in both groups (Figure 2). Similarly, survival was worse for the patients receiving a single-lung transplant compared to patients receiving a double-lung transplant in either of the groups (Figure 3).
Postoperative complications
The ECMO group experienced a significantly higher rate of postoperative complications, including the need for blood transfusions (34.3% versus 4.4%, p ≤ 0.01), airway dehiscence (4.2% versus 1.3%, p ≤ 0.01), stroke (2.8% versus 1.9%, p ≤ 0.01), need for dialysis (31.5% versus 5.8%, p ≤ 0.01), infections requiring antibiotics (55.9% versus 42.4%, p ≤ 0.01), pulmonary embolisms 9.8% versus 0.6%, p ≤ 0.01), and the need for other posttransplant surgical procedures (60.8% versus 19.1%, p ≤ 0.01) (Table 6). ECMO patients tended to have a slightly higher rate of acute rejection episodes posttransplant 10.5% versus 6.9%, though this was not found to be statistically significant (p = 0.09) (Tables 7–8).
| ECMO group (n = 143) | No ECMO group (n = 14,120) | p-value | |
|---|---|---|---|
| Airway dehiscence | 6 (4.2%) | 186 (1.3%) | <0.0001 |
| Stroke (posttransplant) | 4 (2.8%) | 265 (1.9%) | <0.0001 |
| Dialysis (posttransplant) | 45 (31.5%) | 821 (5.8%) | <0.0001 |
| Any drug treated infection (posttransplant) | 29 (56.9%) | 3360 (42.4%) | <0.0001 |
| Other surgical procedures (posttransplant) | 31 (60.8%) | 1516 (19.1%) | <0.0001 |
| Pulmonary embolism after listing | 5 (9.8%) | 46 (0.6%) | <0.0001 |
| Transfusions since listing (Yes) | 49 (34.3%) | 622 (4.4%) | <0.0001 |
| ECMO group (n = 143) | No ECMO group (n = 14,120) | p-value | |
|---|---|---|---|
| Patient/graft status | |||
| Alive | 52 (36.4%) | 7093 (50.2%) | 0.0055 |
| Dead | 86 (60.1%) | 6439 (45.6%) | |
| Lost | 2 (1.4%) | 147 (1.0%) | |
| Retransplanted | 3 (2.1%) | 441 (3.1%) | |
| Graft status | n = 131 | n = 12781 | |
| Functioning | 96 (73.3%) | 10766 (84.2%) | 0.0006 |
| Failed | 35 (26.7%) | 2015 (15.8%) | |
| Primary cause of death (most common) | n = 86 | n = 6409 | 0.0197 |
| Unknown | 3 (3.5%) | 403 (6.3%) | |
| Other | 2 (2.3%) | 366 (5.7%) | |
| Primary graft failure | 16 (18.6%) | 310 (4.8%) | |
| Hyperacute graft failure | 1 (1.2%) | 14 (0.2%) | |
| Chronic rejection graft failure | 5 (5.8%) | 795 (12.4%) | |
| Graft failure due to infection | 1 (1.2%) | 38 (0.59%) | |
| Bacterial septicemia | 10 (11.6%) | 490 (7.7%) | |
| CMV infection | 0 (0%) | 57 (0.9%) | |
| Bacterial pneumonia | 2 (2.33%) | 413 (6.4%) | |
| Fungal infection (Aspergillus) | 3 (3.5%) | 123 (1.9%) | |
| Infection (other) | 2 (2.3%) | 83 (1.3%) | |
| Cardiac arrest | 4 (4.7%) | 221 (3.5%) | |
| Ventricular failure | 2 (2.3%) | 22 (0.3%) | |
| Cardiovascular (other) | 2 (2.3%) | 47 (0.7%) | |
| Respiratory failure | 3 (3.5%) | 820 (12.8%) | |
| ARDS | 1 (1.2%) | 80 (1.3%) | |
| Pulmonary (other) | 1 (1.2%) | 99 (1.5%) | |
| Stroke | 1 (1.2%) | 76 (1.2%) | |
| Metastatic disease (other) | 3 (3.5%) | 183 (2.9%) | |
| Multiple organ failure | 8 (9.3%) | 338 (5.3%) | |
| Graft failure cause | n = 35 | n = 1997 | <0.0001 |
| Primary nonfunction | 24 (68.6%) | 537 (26.9%) | |
| Acute rejection | 1 (2.9%) | 105 (5.3%) | |
| Chronic rejection | 8 (22.9%) | 1065 (53.3%) | |
| Other | 2 (5.7%) | 290 (14.5%) | |
DISCUSSION
In this study, we analyzed the UNOS database to compare the outcomes of patients who were bridged to lung transplantation with ECMO to recipients who were not over an 11-year span. We believe this to be the largest multicenter cohort of ECMO-bridged lung transplant recipients to date. Our analysis suggests that there is no survival benefit afforded to lung transplant recipients bridged with ECMO. Contrary to many recently published studies, recipients bridged with ECMO fared very poorly with a third dying within the first month and barely attaining double-digit 5-year survival rates (11%).
From a different perspective, however, the ECMO-bridged group was comprised of much sicker patients, more often requiring some form of life support. Furthermore, they had higher lung allocation scores, longer graft ischemia times, and were more likely to have had prior lung surgery (nontransplant), while on the waiting list. Finally, many of the ECMO-supported patients had refractory and severe pulmonary hypertension as indicated by the use of both inhaled and infused prostacyclins as well as nitric oxide (Table 9). This likely represents a risk factor for poor outcomes after lung transplantation [7]. In practical terms, most of these transplants should be considered “salvage” attempts. Indeed, the double-salvage strategy of using ECMO for primary graft failure followed by another lung transplant lead to dismal outcomes (Figure 2).
| ECMO group (n = 105) | No ECMO group (n = 1034) | p-value | |
|---|---|---|---|
| Intraortic balloon pump | 2 (1.4%) | 4 (0.03%) | <0.0001 |
| Prostacyclin infusion | 3 (2.1%) | 76 (0.54%) | 0.0124 |
| Prostacyclin inhalation | 1 (0.7) | 12 (0.08%) | 0.0154 |
| IV inotropes | 1 (0.7%) | 0 (0%) | <0.0001 |
| Inhaled nitric oxide | 17 (11.9%) | 25 (0.2%) | <0.0001 |
| Ventilator support | 73 (51.1%) | 600 (4.3%) | <0.0001 |
| Other life support | 8 (5.6%) | 317 (2.3%) | 0.0076 |
We also found that ECMO-bridged lung transplant recipients experienced a higher rate of posttransplant complications, including airway dehiscence, stroke, need for posttransplant dialysis, serious infections, pulmonary embolism, blood transfusion requirements, and the need for another surgical procedure. Although some of these complications can be attributed to the sicker nature of these patients, others may be direct complications of ECMO itself. Naturally, some exceptionally good results with ECMO bridging have been achieved in a handful of transplant centers [1–6].
Our study has some inherent limitations. First, the database is limited to the amount of detail that is provided for the ECMO-bridged recipients. It does not differentiate between veno-venous and veno-arterial ECMO. Nor does it specify the length of time that patients were on ECMO support. The database also has missing data; therefore, our analysis was limited to the variables with enough data. Despite these limitations, the survival outcomes are clear. Second, there are certain important questions that this study was not able to address, including the optimal time to institute ECMO support and when bridged patients should be deactivated while on the waiting list. Third, although the ECMO-bridged recipient cohort in the present study is the largest published to date, it is a relatively small (i.e. 143 patients), compared to the nonbridged group. Despite recent publications [3–4] proposing that ECMO is safer owing to improved technologies, it is likely that better selection of patients for ECMO has also contributed to better outcomes.
Our analysis confirms that salvage ECMO for primary graft dysfunction/acute rejection followed by another lung transplant is associated with extremely poor outcomes. This finding has important implications, given the scarcity of donor lungs, we suggest that firm criteria for instituting salvage ECMO should be instituted.
Although outcomes associated with ECMO in adult patients have improved over time, its use as a bridging strategy in the sickest of lung transplant recipients has yielded relatively poor survival rates and should be carefully scrutinized with prospective trials.