- Record: found
- Abstract: found
- Article: found
Update on the diagnosis and treatment of neuromyelitis optica spectrum disorders (NMOSD) – revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part II: Attack therapy and long-term management
Read this article at
Abstract
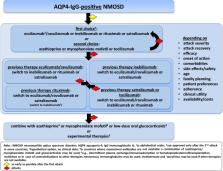
This manuscript presents practical recommendations for managing acute attacks and implementing preventive immunotherapies for neuromyelitis optica spectrum disorders (NMOSD), a rare autoimmune disease that causes severe inflammation in the central nervous system (CNS), primarily affecting the optic nerves, spinal cord, and brainstem. The pillars of NMOSD therapy are attack treatment and attack prevention to minimize the accrual of neurological disability. Aquaporin-4 immunoglobulin G antibodies (AQP4-IgG) are a diagnostic marker of the disease and play a significant role in its pathogenicity. Recent advances in understanding NMOSD have led to the development of new therapies and the completion of randomized controlled trials. Four preventive immunotherapies have now been approved for AQP4-IgG-positive NMOSD in many regions of the world: eculizumab, ravulizumab - most recently-, inebilizumab, and satralizumab. These new drugs may potentially substitute rituximab and classical immunosuppressive therapies, which were as yet the mainstay of treatment for both, AQP4-IgG-positive and -negative NMOSD. Here, the Neuromyelitis Optica Study Group (NEMOS) provides an overview of the current state of knowledge on NMOSD treatments and offers statements and practical recommendations on the therapy management and use of all available immunotherapies for this disease. Unmet needs and AQP4-IgG-negative NMOSD are also discussed. The recommendations were developed using a Delphi-based consensus method among the core author group and at expert discussions at NEMOS meetings.
Related collections
Most cited references256
- Record: found
- Abstract: found
- Article: not found
International consensus diagnostic criteria for neuromyelitis optica spectrum disorders
- Record: found
- Abstract: found
- Article: found