- Record: found
- Abstract: found
- Article: found
Plasmodium microtubule-binding protein EB1 is critical for partitioning of nuclei in male gametogenesis
Read this article at
ABSTRACT
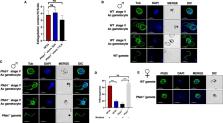
Sexual reproduction of the malaria parasites is critical for their transmission to a mosquito vector. Several signaling molecules, such as kinases and phosphatases, are known to regulate this process. We previously demonstrated that Plasmodium falciparum ( Pf) Ca 2+-dependent protein kinase 4 (CDPK4) and serine/arginine-rich protein kinase 1 (SRPK1) are critical for axoneme formation during male gametogenesis, with genetic deletion of either gene causing a complete block in parasite transmission to the mosquito. A comparative phospho-proteome analysis of Pfcdpk4 − and RNA-seq analysis of Pfsrpk1 − gametocytes showed that these kinases regulate similar biological processes linked to both microtubule (MT) dynamics and cell motility. One of these proteins was a nuclear MT-associated End Binding protein 1 (EB1), which was hypophosphorylated in Pfcdpk4 − gametocytes. To study the functional relevance of EB1, we created gene deletion parasites for EB1. We further demonstrate that Pfeb1 − parasites like WT NF54 parasites proliferate normally as asexuals and undergo gametocytogenesis and gametogenesis. Strikingly, these parasites suffer a severe defect in nuclear segregation and partitioning of nuclei into emerging microgametes. Further genetic crosses utilizing male- and female-sterile parasites revealed that Pfeb1 − parasites only suffer a male fertility defect. Overall, our study reveals an essential function for PfEB1 in male gamete nuclear segregation and suggests a potential therapeutic avenue in the design of transmission-blocking drugs to prevent malaria transmission from humans to mosquito.
IMPORTANCE
Gametogenesis and subsequent gamete fusion are central to successful transmission of the malaria parasites to a female Anopheles mosquito vector and completion of the sexual phase of the parasite life cycle. Male gametogenesis involves the formation of axonemes inside male gametes from male gametocytes via active cytoskeleton remodeling. The tubulin and tubulin-binding proteins are, thus, attractive anti-malarial drug targets. In the present study, we demonstrate that a microtubule-binding protein PfEB1 is essential for male gamete fertility, specifically for the inheritance of nuclei from activated male gametocytes. Targeting PfEB1 function may provide new avenues into designing interventions to prevent malaria transmission and disease spread.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
Cell mechanics and the cytoskeleton.
- Record: found
- Abstract: found
- Article: not found
Actin and Actin-Binding Proteins.
- Record: found
- Abstract: found
- Article: not found