- Record: found
- Abstract: found
- Article: found
Hypercompact CRISPR–Cas12j2 (CasΦ) enables genome editing, gene activation, and epigenome editing in plants
review-article
Shishi Liu
1
,
5 ,
Simon Sretenovic
2
,
5 ,
Tingting Fan
1
,
5 ,
Yanhao Cheng
2 ,
Gen Li
2 ,
Aileen Qi
2 ,
Xu Tang
1 ,
Yang Xu
1 ,
Weijun Guo
3 ,
Zhaohui Zhong
1 ,
Yao He
1 ,
Yanling Liang
1 ,
Qinqin Han
1 ,
Xuelian Zheng
1 ,
Xiaofeng Gu
3 ,
Yiping Qi
2
,
4
,
∗ ,
Yong Zhang
1
,
∗∗
20 September 2022
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
CRISPR-Cas9, -Cas12a, -Cas12b, and -Cas13 have been harnessed for genome engineering
in human and plant cells (Liu et al., 2022). However, the large size of these Cas
proteins (e.g. ∼190 kDa for SpCas9) makes them difficult to deliver into cells via
a viral vector. The development of smaller Cas proteins will lead to reduced viral
vector sizes that can be more widely adopted in versatile genome engineering systems.
Recently, a CRISPR-Cas12j2 (CasΦ) system was discovered in huge phages and developed
into a hypercompact genome editor due to the small size of Cas12j2 (∼80 kDa) (Pausch
et al., 2020). Unfortunately, the gene editing efficiency of Cas12j2 in Arabidopsis
protoplasts using ribonucleoprotein delivery was less than one percent (Pausch et al.,
2020). Further optimization of this system is clearly required if CRISPR-Cas12j2-mediated
editing in plant genomes is to be adopted by the plant sciences community.
To develop an efficient CRISPR-Cas12j2 genome editing system in plants, we used an
efficient dual Pol II promoter DNA-based expression system previously applied for
CRISPR-Cas12a (Tang et al., 2017) and CRISPR-Cas12b (Ming et al., 2020). In this system,
the crRNA is processed by HH and HDV ribozymes (Figure 1A). Cas12j2 prefers T-rich
protospacer adjacent motifs (PAMs) according to PAM-depletion assays in bacteria (Pausch
et al., 2020). To assess the PAM requirements of Cas12j2 in plants, we selected 17
protospacer sequences that were each present twice in the rice genome but contained
slightly different PAMs: VTTV or VTTTV. Rice protoplast assays demonstrated that about
half of the 34 target sites exhibited genome edits as determined by next-generation
sequencing (NGS) with an efficiency of up to 40% (Figure 1B). Strikingly, on most
occasions, the target sites with the VTTV/VTTTV PAM pairs of the same protospacer
sequence showed similar editing tendency (Figure 1B), suggesting important roles of
protospacer sequences in Cas12j2-mediated genome editing. The VTTV PAM was clearly
preferred by Cas12j2 over the VTTTV PAM (Figure 1B). We next investigated the importance
of the flanking “V” nucleotides in the VTTV PAM by testing 35 target sites in rice
cells. The majority of target sites containing NTTA, NTTC, and NTTG PAMs showed detectable
genome editing (up to 25%) whereas most NTTT PAM sites showed no editing (Figure 1C).
Targeting protospacer sequences with four non-canonical TSN (S = G or C) PAM sites
(Pausch et al., 2020) exhibited minimal editing activity (Supplemental Figure 1).
These data collectively support that NTTV is the preferred PAM of Cas12j2 in plants.
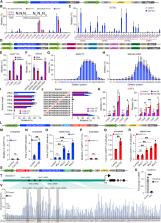
Figure 1
Development of the CRISPR–Cas12j2 system for genome editing, gene activation, and
DNA methylation-based gene silencing in plants.
(A) Schematic of the dual RNA polymerase II promoter system for Cas12j2 and crRNA
expression.
(B) Assessment of the effects of PAM and protospacer sequence on genome editing efficiency
with CRISPR-Cas12j2 at 34 target sites in rice protoplasts.
(C) Refined analysis of NTTN PAM requirements for CRISPR-Cas12j2 in rice protoplasts.
(D) Schematics of the dual RNA polymerase II promoter-based and multiplexed CRISPR-Cas12j2
systems for genome editing in rice and tomato, respectively.
(E) Multiplexed editing of four target sites in rice protoplasts.
(F) Multiplexed editing of four target sites in tomato protoplasts.
(G) Deletion position profile for a representative target site in rice.
(H) Deletion position profile for a representative target site in tomato.
(I) Assessment of protospacer length requirements at two PAM sites with the same protospacer
in rice protoplasts (N.D., not detected).
(J) Assessment of targeting specificity using mismatched crRNAs at two PAM sites with
the same protospacer in rice protoplasts.
(K) Improvement of genome editing efficiency using engineered Cas12j2 variants.
(L) Schematic of a CRISPR-Cas12j2 transcriptional activation system.
(M–R) Transcriptional activation of OsER1 and OsNRT1.1A in rice.
(M and P) Testing of genome editing efficiency.
(N and Q) Testing of transcriptional activation in rice protoplasts.
(O and R) Testing of transcriptional activation in transgenic rice lines.
(S) Schematic of a CRISPR-Cas12j2-based targeted DNA methylation system.
(T) Schematic of targeted DNA methylation of the OsGBSS1 promoter. Four target sites
are indicated by black arrow heads. The three regions chosen for bisulfite sequencing
are indicated by green arrows.
(U) Targeted gene silencing of OsGBSS1 with directed DNA methylation in rice protoplasts.
(V) DNA methylation at target regions determined using bisulfate sequencing of PCR
products. For experiments in rice and tomato protoplasts, three biological replicates
were used. The error bars denote standard deviations. Asterisks are used to denote
statistical significance by Student’s t-test (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001;
ns, not significant).
We next turned our attention to developing a multiplexed Cas12j2 genome editing system using
the RNA polymerase II promoter and HH-HDV ribozyme systems (Ming et al., 2020; Zhang
et al., 2021) (Figure 1D). Multiplexed editing at four select sites showed high genome
editing efficiency (∼15% to ∼50%) in rice protoplasts (Figure 1E). A total of 16 VTTV
PAM sites in the tomato genome were targeted by four multiplexed Cas12j2 constructs.
Genome editing was detected at 12 out of 16 sites with variable efficiencies (Figure 1F
and Supplemental Figure 2). In both rice and tomato cells, the editing outcomes were
deletions nearly every time (Figure 1E-F and Supplemental Figure 2). In both rice
and tomato, these deletions were frequently found at target bases distal to the PAM
(Figure 1G-H and Supplemental Figure 3). Deletion sizes were typically 7–13 bp (Supplemental
Figure 4). These results demonstrate that staggered cleavage is created by Cas12j2
as previously observed with Cas12a and Cas12b (Zetsche et al., 2015; Tang et al.,
2017; Ming et al., 2020).
To assess the specificity of Cas12j2, we used a single high-activity protospacer that
has two target sites in the rice genome differing by a VTTV or VTTTV PAM site. Protospacers
with lengths of 24 (only with the VTTV PAM), 22, 20 (the default protospacer length),
18, 16, and 14 nucleotides were used. The results showed that protospacers of 18 bp
or longer resulted in optimal editing efficiencies, whereas 16 bp and 14 bp protospacers
resulted in reduced and undetectable editing, respectively (Figure 1I). Permutations
of every two nucleotides across the 20 bp protospacer demonstrated that mutations
at PAM-distal sites could be well tolerated (Figure 1J). However, simultaneous introduction
of two adjacent mutations at positions 1–14 of the protospacer completely abolished
the nuclease activity of Cas12j2 (Figure 1J). These data demonstrate that Cas12j2
is a small but highly specific nuclease.
Two Cas12j2 variants, nCas12j2 and vCas12j2, were previously shown to have enhanced
in vitro nuclease kinetics over Cas12j2 (Pausch et al., 2021). We tested both variants
on 15 target sites in rice protoplasts and found that nCas12j2 had significantly improved
editing activity at 6 out of 15 target sites while vCas12j2 had similar activity to
the wild-type Cas12j2 (Figure 1K and Supplemental Figure 5). These results suggest
that in vivo genome editing by Cas12j2 can be improved through protein engineering and
that nCas12j2 is better suited for genome editing in plants and likely other eukaryotic
cells.
The improved nCas12j2 was next tested for its ability to perform genome edits in whole
transgenic plants. Low genome editing efficiencies of 1.5%, 2.5%, 6%, and 20% were
detected by NGS among 39 rice lines generated with four T-DNA constructs (Supplemental
Figure 6A–D). This contrasts with the relatively higher editing efficiencies in rice
protoplasts. qRT–PCR analysis of select T0 lines showed that the expression level
of Cas12j2 was relatively high (Supplemental Figure 6E), which suggests that low genome
editing efficiency in stable lines was not due to low Cas12j2 expression. We next
generated four multiplexed Cas12j2 constructs for editing 16 target sites in four
genes from poplar (four sites per gene). An analysis of 16 T0 transgenic plants per
construct revealed very low editing efficiencies (up to 1.2%) at all target sites
(Supplemental Figure 7A–D) even though Cas12j2 expression was normal (Supplemental
Figure 7E). These data point to a disconnect between Cas12j2-mediated genome editing
in stable lines versus protoplasts. To solve this puzzle, we selected three T0 rice
lines that express nCas12j2 and crRNAs for editing the AG17–TT site. Although we could
not detect successful genome editing in the leaves of these seedlings by Sanger sequencing
(Supplemental Figure 8A), we did detect genome editing efficiencies of roughly 50%
in protoplasts derived from these seedlings after 48 h or 72 h of resting (Supplemental
Figure 8B-C). These data suggest that it may be more efficient to obtain nCas12j2-edited
plants via protoplast regeneration rather than stable plant transformation (Yue et al.,
2021).
To engineer a CRISPR-Cas12j2 system that can activate the transcription of target
genes, we fused a potent TV (6TAL–VP128) activation domain (Li et al., 2017) to the
C-terminus of Cas12j2 (Figure 1L). The resulting Cas12j2 activator was tested for
transcriptional activation of OsER1 and OsNRT1.1A using 16 bp protospacers in rice
protoplasts. While no edits were detected in either gene, four- and two-fold increases
in gene expression were observed for OsER1 and OsNRT1.1A, respectively (Figure 1M-P).
Even higher gene expression was detected in stable transgenic plants: a ten-fold increase
for OsER1 and a four-fold increase for OsNRT1.1A (Figure 1O and Figure 1R). We further
demonstrated that two additional target genes (OsCHS and OsGBSS1) could be transcriptionally
activated by Cas12j2 without being edited (Supplemental Figure 9A-B).
Finally, a CRISPRoff configuration was adopted to develop a Cas12j2-derived epigenome
editing system for targeted DNA methylation in plants (Nunez et al., 2021) (Figure 1S).
Four crRNAs were designed for multiplexed targeting of the promoter region of OsGBSS1
(Figure 1T). Transformation of the Cas12j2 epigenome editor (5mC) in rice cells resulted
in a drastic reduction of the mRNA level for OsGBSS1, which coincided with methylation
of the promoter as determined by bisulfite sequencing (Figure 1U-V).
In summary, we demonstrate that the hypercompact Cas12j2 can be used for genome editing
in a variety of plant species. Furthermore, we repurposed Cas12j2 for gene activation
and epigenome editing to fine-tune target gene expression in plants. Our observation
that Cas12j2 has a unique preference for editing in non-dividing cells warrants further
investigation to determine the exact mechanism by which this occurs. Furthermore,
we repurposed Cas12j2 for gene activation and epigenome editing to fine-tune target
gene expression in plants. With further improvement of Cas12j2, we anticipate that
this small Cas protein will become widely adopted for versatile applications in plant
genome engineering (Lyu, 2020).
Funding
This work was supported by the National Key Research and Development Program of China
(award no. NK2022010204) to Y.Z.; the National Natural Science Foundation of China
(award nos. 32270433, 32101205, 32072045, and 31960423) to X.T., X.Z., and Y.Z.; the
Sichuan Science and Technology Program (award no. 2021JDRC0032) to Y.Z.; and the Technology
Innovation and Application Development Program of Chongqing (award no. CSTC2021JSCX-CYLHX0001)
to X.T. and Y.Z. This work is also supported by the National Science Foundation Plant
Genome Research Program grant (award nos. IOS-1758745 and IOS-2029889) and USDA-AFRI
Agricultural Innovations Through Gene Editing Program (award no. 2021-67013-34554)
to Y.Q. S.S. is a fellow of the Foundation for Food and Agriculture Research.
Author contributions
Y.Z. and Y.Q. designed the experiments. S.L. and S.S. designed and made the constructs.
S.L., S.S., T.F., X.T., A.Q., Y.X., and Z.Z. generated all T-DNA vectors. S.L. and
T.F. conducted rice protoplast isolation and transformation. S.L., Y.X., Y.H., Y.L.,
Q.H., and X.Z. did NGS analysis for genome editing in rice protoplasts and stable
lines. W.G. and X.G. analyzed the rice methylation NGS data. Y.C. and S.S. did the
tomato protoplast transformation and NGS analysis of editing. G.L. conducted stable
transformation of poplar and NGS analysis of editing. Y.Z. and Y.Q. analyzed the data
and wrote the manuscript. All authors participated in discussion and revision of the
manuscript.
Related collections
Most cited references11
- Record: found
- Abstract: found
- Article: not found
Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system.
- Record: found
- Abstract: found
- Article: not found
CRISPR-CasΦ from huge phages is a hypercompact genome editor
Patrick Pausch, Basem Al-Shayeb, Ezra Bisom-Rapp … (2020)
- Record: found
- Abstract: not found
- Article: not found
Genome-wide programmable transcriptional memory by CRISPR-based epigenome editing
James K. Nuñez, Jin Shan Chen, Greg C. Pommier … (2021)