- Record: found
- Abstract: found
- Article: found
Stem cell-derived CAR T cells show greater persistence, trafficking, and viral control compared to ex vivo transduced CAR T cells

Read this article at
Abstract
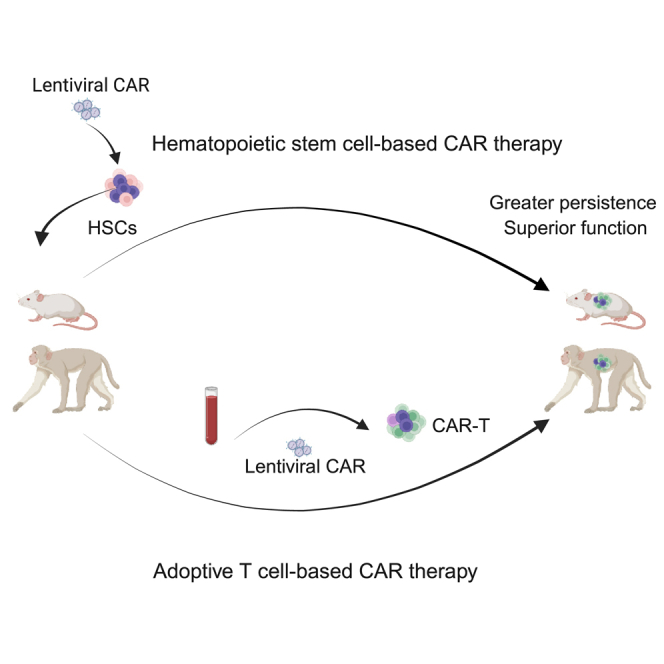
Adoptive cell therapy (ACT) using T cells expressing chimeric antigen receptors (CARs) is an area of intense investigation in the treatment of malignancies and chronic viral infections. One of the limitations of ACT-based CAR therapy is the lack of in vivo persistence and maintenance of optimal cell function. Therefore, alternative strategies that increase the function and maintenance of CAR-expressing T cells are needed. In our studies using the humanized bone marrow/liver/thymus (BLT) mouse model and nonhuman primate (NHP) model of HIV infection, we evaluated two CAR-based gene therapy approaches. In the ACT approach, we used cytokine enhancement and preconditioning to generate greater persistence of anti-HIV CAR + T cells. We observed limited persistence and expansion of anti-HIV CAR T cells, which led to minimal control of the virus. In our stem cell-based approach, we modified hematopoietic stem/progenitor cells (HSPCs) with anti-HIV CAR to generate anti-HIV CAR T cells in vivo. We observed CAR-expressing T cell expansion, which led to better plasma viral load suppression. HSPC-derived CAR cells in infected NHPs showed superior trafficking and persistence in multiple tissues. Our results suggest that a stem cell-based CAR T cell approach may be superior in generating long-term persistence and functional antiviral responses against HIV infection.
Graphical abstract
Abstract
Kitchen and colleagues use hematopoietic stem cells and T cells to generate anti-HIV CAR T cells and evaluate the efficacy of each approach against HIV infection. Stem-based CAR T cells show superior persistence, trafficking, and viral suppression compared to conventional ex vivo CAR-modified T cells in HIV- and SHIV-infected animals.
Related collections
Most cited references82
- Record: found
- Abstract: found
- Article: not found
Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma
- Record: found
- Abstract: found
- Article: not found
Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia
- Record: found
- Abstract: found
- Article: not found