- Record: found
- Abstract: found
- Article: found
Mapping the Follicle-Stimulating Hormone-Induced Signaling Networks
Read this article at
Abstract
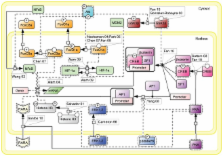
Follicle-stimulating hormone (FSH) is a central regulator of male and female reproductive function. Over the last decade, there has been a growing perception of the complexity associated with FSH-induced cellular signaling. It is now clear that the canonical Gs/cAMP/PKA pathway is not the sole mechanism that must be considered in FSH biological actions. In parallel, consistent with the emerging concept of biased agonism, several examples of ligand-mediated selective signaling pathway activation by gonadotropin receptors have been reported. In this context, it is important to gain an integrative view of the signaling pathways induced by FSH and how they interconnect to form a network. In this review, we propose a first attempt at building topological maps of various pathways known to be involved in the FSH-induced signaling network. We discuss the multiple facets of FSH-induced signaling and how they converge to the hormone integrated biological response. Despite of their incompleteness, these maps of the FSH-induced signaling network represent a first step toward gaining a system-level comprehension of this hormone’s actions, which may ultimately facilitate the discovery of novel regulatory processes and therapeutic strategies for infertility and non-steroidal contraception.
Related collections
Most cited references111
- Record: found
- Abstract: found
- Article: not found
Transduction of receptor signals by beta-arrestins.
- Record: found
- Abstract: found
- Article: not found
Teaching old receptors new tricks: biasing seven-transmembrane receptors.
- Record: found
- Abstract: found
- Article: not found