- Record: found
- Abstract: found
- Article: found
Genomic Characterization of ESBL/AmpC-Producing Escherichia coli in Stray Dogs Sheltered in Yangzhou, China
Read this article at
Abstract
Purpose
Limited data are available on the prevalence and antimicrobial resistance of extended spectrum β-lactamase- (ESBL) and AmpC β-lactamase-producing Escherichia coli in stray dogs. We aimed to investigate the genomic characteristics of ESBL/AmpC-producing E. coli isolated from stray dogs sheltered in Yangzhou, China.
Methods
We collected 156 samples including 115 fecal swabs, 35 kennel floor swabs, two breeder hand and shoe sole swabs, and four feed samples. The isolates were tested for resistance by antimicrobial susceptibility testing and further analyzed for cefotaxime-resistant E. coli isolates by whole genome sequencing.
Results
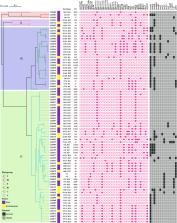
We identified 80 cefotaxime-resistant E. coli isolates (51.3%), 59 isolates (73.8%) from feces and 21 (26.2%) from the environment. Whole-genome sequencing analysis showed that bla CTX-M-15 (n=30) and bla CTX-M-55 (n=29) were the most prevalent genotypes. Two isolates only carried the AmpC β-lactamase gene bla CMY-2; one isolate had a combination of AmpC β-lactamase gene bla DHA-1 and ESBL β-lactamase gene bla CTX-M-14. Other important resistance genes such as bla OXA-10, bla TEM-1B, bla TEM-135, bla TEM-106, tet(A), qnrS1, qnrB4, and oqxAB were also detected. The serotype combination was highly abundant, with O10:H25 predominating (n=12). Most cefotaxime-resistant E. coli isolates belonged to phylogroup A (62.5%, n=50), followed by phylogroup B1 (26.3%, n=21). Thirty different sequence types (STs) and 27 distinct plasmid replicons were identified, among which ST2325 (n=12) and IncFII (n=38) was the most frequent ST and plasmid, respectively. ESBL/AmpC-producing isolates were divided into four major clades; clade IV was the primary lineage containing 37 isolates from feces and 13 from the environment. Three high-risk E. coli clone ST23 strains and one ST10 strain belonged to clades III and IV, respectively.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing.

- Record: found
- Abstract: found
- Article: found
The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes
- Record: found
- Abstract: found
- Article: not found