- Record: found
- Abstract: found
- Article: found
Cardioprotective effects of miR-34a silencing in a rat model of doxorubicin toxicity
Read this article at
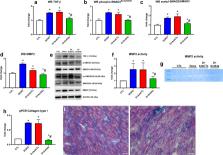
Abstract
Cardiotoxicity remains a serious problem in anthracycline-treated oncologic patients. Therapeutic modulation of microRNA expression is emerging as a cardioprotective approach in several cardiovascular pathologies. MiR-34a increased in animals and patients exposed to anthracyclines and is involved in cardiac repair. In our previous study, we demonstrated beneficial effects of miR-34a silencing in rat cardiac cells exposed to doxorubicin (DOXO). The aim of the present work is to evaluate the potential cardioprotective properties of a specific antimiR-34a (Ant34a) in an experimental model of DOXO-induced cardiotoxicity. Results indicate that in our model systemic administration of Ant34a completely silences miR-34a myocardial expression and importantly attenuates DOXO-induced cardiac dysfunction. Ant34a systemic delivery in DOXO-treated rats triggers an upregulation of prosurvival miR-34a targets Bcl-2 and SIRT1 that mediate a reduction of DOXO-induced cardiac damage represented by myocardial apoptosis, senescence, fibrosis and inflammation. These findings suggest that miR-34a therapeutic inhibition may have clinical relevance to attenuate DOXO-induced toxicity in the heart of oncologic patients.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Cardiac Fibrosis: The Fibroblast Awakens.
- Record: found
- Abstract: found
- Article: not found
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.
- Record: found
- Abstract: found
- Article: not found