- Record: found
- Abstract: found
- Article: found
Treatment effects after maxillary expansion using invisalign first system vs. acrylic splint expander in mixed dentition: a prospective cohort study
Read this article at
Abstract
Background
Invisalign First System (First) is a new type of orthodontic appliance for maxillary arch expansion in mixed dentition children. Till now, few studies have evaluated the expansion effects of First versus other appliances. What’s more, most studies of arch expansion did not include a natural group to rule out growth effects. This prospective cohort study aimed to evaluate the dental and dentoalveolar effects using First or acrylic splint rapid maxillary expander (RME) in adolescents excluding growth factors.
Materials and methods
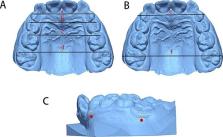
After screening by strict inclusion criteria and propensity score matching (PSM), fifty-one patients were included: First group (n = 17), RME group (n = 17), and natural growth (NG) group (n = 17). Nine indicators including dental arch width, dentoalveolar arch width, and inclination of the molars were measured on digital dental casts at baseline (T0) and six-month follow-up (T1). Paired t-tests were used for intra-group results, and two-sample independent t-tests were used for inter-group comparisons.
Results
There was no significant increase in all indicators within six months in the NG group ( p > 0.05). In the First group and RME group, all width indicators were significantly increased after treatment ( p < 0.05). The RME group exhibited greater expansion than the First group in intercanine width, first interpremolar width, second interdeciduous molar width, first intermolar width, arch perimeter, intercanine dentoalveolar width, intermolar dentoalveolar width, and inclination of the molars ( p < 0.05). Whereas, there was no significant difference in arch depth between the two treated groups.
Conclusions
Both First and RME can expand the maxillary arch in mixed dentition. In case of mild to moderate maxillary transverse deficiency (MTD), Invisalign First System could be a reasonable option. RME shows significant better efficiency of dental arch expansion than First, recommended for patients with severe MTD.
Trial registration
This prospective study was registered on ClinicalTrials.gov (01/02/2022, registration number: ChiCTR2200056220). The trial was approved by the Ethical Committee of the Hunan Xiangya Stomatological Hospital Central South University (20,200,088), and informed consent was obtained from all subjects and their legal guardian(s).
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003.
- Record: found
- Abstract: not found
- Article: not found
Matching Using Estimated Propensity Scores: Relating Theory to Practice

- Record: found
- Abstract: found
- Article: found