- Record: found
- Abstract: found
- Article: found
Suboptimal Uptake, Retention, and Adherence of Daily Oral Prexposure Prophylaxis Among People With Opioid Use Disorder Receiving Hepatitis C Virus Treatment
Read this article at
Abstract
Background
Daily oral preexposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) prevents human immunodeficiency (HIV) among people who inject drugs (PWID). Despite rising HIV incidence and injection drug use (IDU), PrEP use remains low and there is limited research about uptake, adherence, and retention among PWID.
Methods
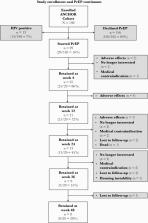
The ANCHOR investigation evaluated a community-based care model collocating hepatitis C virus (HCV) treatment, medication for opioid use disorder (OUD), and PrEP in individuals in Washington, DC, and Baltimore, Maryland. PrEP counseling was conducted from HCV treatment day 0 until week 24. Subjects could start any time during this window, were followed for 48 weeks, and were assessed for adherence by self-report and dried blood spot TDF analysis.
Results
One hundred ninety-eight participants were enrolled, of whom 185 (93%) were HIV negative. Twenty-nine individuals (15.7% of HIV-negative cohort) initiated PrEP. One hundred sixteen participants (62.7%) met 2014 Centers for Disease Control and Prevention (CDC) PrEP criteria due to IDU (82 [44.3%]), sex (9 [4.9%]), or both practices (25 [13.5%]). Providers recommended PrEP to 94 individuals (50.8%), and recommendation was associated with PrEP uptake. Median treatment duration was 104 days (interquartile range, 28–276 days), with 8 participants retained through week 48. Adherence was variable over time by self-report and declined by TDF analysis. No HIV seroconversions occurred.
Conclusions
This cohort of people with HCV and OUD experienced low uptake of PrEP despite the majority meeting CDC criteria. High rates of disruption and discontinuation, compounded by variable adherence, made TDF/FTC a suboptimal prevention strategy. Emerging modalities like long-acting formulations may address these barriers, but PWID have been excluded from their development to date.
Abstract
People with hepatitis C and opioid use disorder experienced low uptake of preexposure prophylaxis despite the majority meeting CDC criteria. High rates of disruption and discontinuation, compounded by variable adherence, made tenofovir disoproxil fumarate/emtricitabine a suboptimal prevention strategy.
Related collections
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection
- Record: found
- Abstract: found
- Article: not found
Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial.
- Record: found
- Abstract: found
- Article: not found