- Record: found
- Abstract: found
- Article: found
MiR-940 promotes malignant progression of breast cancer by regulating FOXO3
Read this article at
Abstract
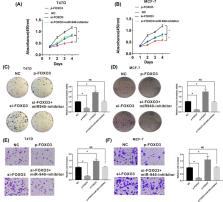
Breast cancer (BC) is a common cancer with poor survival. The present study aimed to explore the effect of miR-940 on the process of BC cells and its target gene FOXO3. The expression of miR-940 was assessed in BC tissues and cells using qRT-PCR. Furthermore, the correlation between miR-940 and prognosis of BC patients from the TCGA database was analyzed. CCK8 assays and colony formation assays were used to explore the effect of miR-940 on BC cell proliferation. The invasion abilities were detected by transwell assays. Luciferase reporter assay was performed to scrutinize the relationship between miR-940 and FOXO3. Finally, rescue experiments were performed through FOXO3 down-regulation and miR-940 inhibitors by using CCK8 assays, colony formation assays and transwell assays. miR-940 was significantly up-regulated in BC cells and tissues. In addition, the high level of miR-940 correlated with poor survival of BC patients ( P=0.023). CCK8 assays, colony formation assays and transwell assays indicated that miR-940 promoted the proliferation and invasion abilities of BC cells. The luciferase reporter assay suggested that miR-940 directly targeted FOXO3. Moreover, we found that the effect of si-FOXO3 was rescued by miR-940 inhibitors in BC cells. miR-940 may promote the proliferation and invasion abilities of BC cells by targeting FOXO3. Our study suggested that miR-940 could be a novel molecular target for therapies against BC.
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
FOXOs, cancer and regulation of apoptosis.

- Record: found
- Abstract: found
- Article: found
Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A
- Record: found
- Abstract: found
- Article: not found