- Record: found
- Abstract: found
- Article: found
Sitafloxacin- Versus Moxifloxacin-Based Sequential Treatment for Mycoplasma Genitalium Infections: Protocol for a Multicenter, Open-Label Randomized Controlled Trial
Abstract
Background
Mycoplasma genitalium is an emerging sexually transmitted pathogen associated with increasing antibiotic resistance. The current treatment guidelines recommend moxifloxacin-sequential therapy for macrolide-resistant M genitalium or strains with unknown resistance profiles. However, it is unclear whether sitafloxacin, a 4th-generation fluoroquinolone antibiotic, is effective against resistant strains.
Objective
This study aims to assess and compare the efficacy and safety of sitafloxacin- and moxifloxacin-based treatment regimens for managing M genitalium infections.
Methods
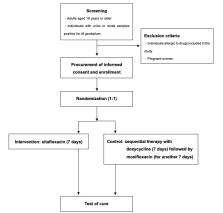
We will conduct this randomized controlled trial at multiple centers in Japan. Eligible participants include adults aged 18 years or older with a confirmed M genitalium infection, as determined through the nucleic acid amplification test. Patients will be randomly assigned using a stratified approach based on the treatment facility and infection site. The interventions comprise oral sitafloxacin (200 mg) daily for 7 days (with optional pretreatment of oral doxycycline, 200 mg, daily for up to 7 days), with a control group receiving oral doxycycline (200 mg) daily for 7 days followed by moxifloxacin (400 mg) daily for another 7 days. The primary outcome is the treatment success rate with a superiority margin of 10%, as confirmed through the nucleic acid amplification test. Secondary outcomes encompass changes in the bacterial load at the urogenital or rectal sites and the emergence of posttreatment-resistant mutant strains.
Results
Enrollment commenced in June 2023 and will conclude in December 2024, with findings anticipated by 2025. The expected success rates fall within the range of 80% for sitafloxacin and 42% for moxifloxacin against M genitalium carrying the G248T (S83I) mutation, based on previous studies. Accordingly, with a 5% significance level (2-sided) and 80% statistical power, we aim to recruit 50 participants per group, factoring in a 10% expected dropout rate.
Conclusions
This study will provide valuable insights into the efficacy and safety of sitafloxacin- versus moxifloxacin-based sequential therapy in treating M genitalium infections. These findings have the potential to influence clinical guidelines, favoring more effective therapeutic choices. The multicenter approach enhances the robustness of this study. However, a limitation is the potential insufficiency of statistical power to detect posttreatment-resistant mutant strains in each group, rendering posttreatment-resistance mutations a notable concern. In the future, we may need to increase the sample size to enhance power.
Related collections
Most cited references32

- Record: found
- Abstract: found
- Article: found
Sexually Transmitted Infections Treatment Guidelines, 2021
- Record: found
- Abstract: found
- Article: not found
Use of TaqMan 5' nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic.
- Record: found
- Abstract: found
- Article: not found