- Record: found
- Abstract: found
- Article: found
Efficacy analysis of three-year subcutaneous SQ-standardized specific immunotherapy in house dust mite-allergic children with asthma
Read this article at
Abstract
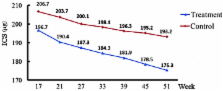
The present study aimed to evaluate the efficacy of three-year subcutaneous SQ-standardized specific immunotherapy (SCIT) in house dust mite (HDM)-allergic children with asthma. Ninety children with allergic asthma to HDMs, with or without allergic rhinitis, were randomly divided into two groups, the treatment group and the control group. The treatment group received SCIT combined with standardized glucocorticoid management and the control group received standardized glucocorticoid management alone for a period of three years. The mean daily dose of inhaled corticosteroids (ICSs), a four-week diary recording the symptom scores of asthma, peak expiratory flow (PEF) measurements, skin prick test results and serum immunoglobulin E (IgE) levels were assessed prior to treatment and following one, two and three years of treatment. The median dose of ICS was reduced in the treatment group after two and three years of treatment compared with that of the control group. After three years of treatment, the discontinuation percentage of ICS in the treatment group was higher than that in the control group. The treatment group demonstrated significantly reduced daytime and night-time asthmatic symptom scores, increased PEF values and reduced serum IgE levels after two and three years of treatment compared with those in the control group (P<0.05). In conclusion, three-year SCIT treatment combined with ICS is an effective immunotherapy for children with allergic asthma and resulted in a reduction of the required ICS dose.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Mechanisms of allergen-specific immunotherapy.
- Record: found
- Abstract: found
- Article: not found
Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial.
- Record: found
- Abstract: found
- Article: not found