- Record: found
- Abstract: found
- Article: found
Autophagy and tight junction proteins in the intestine and intestinal diseases
Read this article at
Abstract
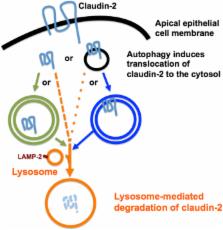
The intestinal epithelium (IE) forms an indispensible barrier and interface between the intestinal interstitium and the luminal environment. The IE regulates water, ion and nutrient transport while providing a barrier against toxins, pathogens (bacteria, fungi and virus) and antigens. The apical intercellular tight junctions (TJ) are responsible for the paracellular barrier function and regulate trans-epithelial flux of ions and solutes between adjacent cells. Increased intestinal permeability caused by defects in the IE TJ barrier is considered an important pathogenic factor for the development of intestinal inflammation, diarrhea and malnutrition in humans and animals. In fact, defects in the IE TJ barrier allow increased antigenic penetration, resulting in an amplified inflammatory response in inflammatory bowel disease (IBD), necrotizing enterocolitis and ischemia-reperfusion injury. Conversely, the beneficial enhancement of the intestinal TJ barrier has been shown to resolve intestinal inflammation and apoptosis in both animal models of IBD and human IBD. Autophagy (self-eating mechanism) is an intracellular lysosome-dependent degradation and recycling pathway essential for cell survival and homeostasis. Dysregulated autophagy has been shown to be directly associated with many pathological processes, including IBD. Importantly, the crosstalk between IE TJ and autophagy has been revealed recently. We showed that autophagy enhanced IE TJ barrier function by increasing transepithelial resistance and reducing the paracellular permeability of small solutes and ions, which is, in part, by targeting claudin-2, a cation-selective, pore-forming, transmembrane TJ protein, for lysosome (autophagy)-mediated degradation. Interestingly, previous studies have shown that the inflamed intestinal mucosa in patients with active IBD has increased claudin-2 expression. In addition, inflammatory cytokines (for example, tumor necrosis factor-α, interleukin-6, interleukin-13, and interleukin-17) whose levels are increased in IBD patients cause an increase in claudin-2 expression and a claudin-2-dependent increase in TJ permeability. Thus, the role of claudin-2 in intestinal pathological processes has been attributed, in part, to the increase of intestinal TJ permeability. Claudin-2 represents a new therapeutic target in treating IBD, diarrhea and malnutrition in animals and humans.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin
- Record: found
- Abstract: found
- Article: not found
Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution.
- Record: found
- Abstract: found
- Article: not found