- Record: found
- Abstract: found
- Article: found
Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells
Read this article at
Abstract
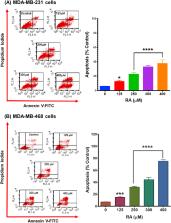
Rosmarinic acid (RA) is a polyphenolic compound with various pharmacological properties, including, anti-inflammatory, immunomodulatory, and neuroprotective, as well as having antioxidant and anticancer activities. This study evaluated the effects and mechanisms of RA in two racially different triple-negative breast cancer (TNBC) cell lines. Results obtained show that RA significantly caused cytotoxic and antiproliferative effects in both cell lines in a dose- and time-dependent manner. Remarkably, RA induced cell cycle arrest-related apoptosis and altered the expression of many apoptosis-involved genes differently. In MDA-MB-231 cells, RA arrested the cells in the G 0/G 1 phase. In contrast, the data suggest that RA causes S-phase arrest in MDA-MB-468 cells, leading to a 2-fold increase in the apoptotic effect compared to MDA-MB-231 cells. Further, in MDA-MB-231 cells, RA significantly upregulated the mRNA expression of three genes: harakiri ( HRK), tumor necrosis factor receptor superfamily 25 ( TNFRSF25), and BCL-2 interacting protein 3 ( BNIP3). In contrast, in the MDA-MB-468 cell line, the compound induced a significant transcription activation in three genes, including TNF, growth arrest and DNA damage-inducible 45 alpha ( GADD45A), and BNIP3. Furthermore, RA repressed the expression of TNF receptor superfamily 11B ( TNFRSF11B) in MDA-MB-231 cells in comparison to the ligand TNF superfamily member 10 ( TNFSF10) and baculoviral IAP repeat-containing 5 (BIRC5) in MDA-MB-468 cells. In conclusion, the data suggest that the polyphenol RA may have a potential role in TNBC therapies, particularly in MDA-MB-468 cells.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains.

- Record: found
- Abstract: found
- Article: found
Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics
- Record: found
- Abstract: found
- Article: not found