- Record: found
- Abstract: found
- Article: found
Drug Development Against Metastatic Cancers
Read this article at
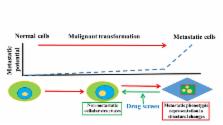
Abstract
While combinational diagnostic and treatment strategies over the past decades have significantly improved the overall survival of cancer patients, metastatic cancer remains a leading cause of death in developed countries. The lack of successful treatment strategies for the disease is in large part due to the complexity of the metastatic transformation, which embodies extensive cellular and extracellular alterations, enabling metastatic cancer cells to reach and colonize other organs. The mode of action for the majority of anti-cancer drugs used in clinics today is primarily tumor growth inhibition. While they are effective in destroying cancer cells, they fall short in blocking metastasis. Here we discuss the evolution of past and current anti-cancer drug development, the limits of current strategies, and possible alternative approaches for future drug development against metastatic cancers.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
RAS oncogenes: the first 30 years.
- Record: found
- Abstract: found
- Article: not found
Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial.
- Record: found
- Abstract: not found
- Article: not found