- Record: found
- Abstract: found
- Article: found
Ecophylogenetics Clarifies the Evolutionary Association between Mammals and Their Gut Microbiota
Read this article at
Abstract
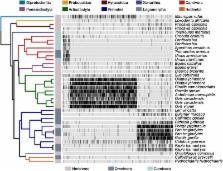
Our understanding of mammalian evolution has become microbiome-aware. While emerging research links mammalian biodiversity and the gut microbiome, we lack insight into which microbes potentially impact mammalian evolution. Microbes common to diverse mammalian species may be strong candidates, as their absence in the gut may affect how the microbiome functionally contributes to mammalian physiology to adversely affect fitness. Identifying such conserved gut microbes is thus important to ultimately assessing the microbiome’s potential role in mammalian evolution. To advance their discovery, we developed an approach that identifies ancestrally related groups of microbes that distribute across mammals in a way that indicates their collective conservation. These conserved clades are presumed to have evolved a trait in their ancestor that matters to their distribution across mammals and which has been retained among clade members. We found not only that such clades do exist among mammals but also that they appear to be subject to natural selection and characterize human evolution.
ABSTRACT
Our knowledge of how the gut microbiome relates to mammalian evolution benefits from the identification of gut microbial taxa that are unexpectedly prevalent or unexpectedly conserved across mammals. Such taxa enable experimental determination of the traits needed for such microbes to succeed as gut generalists, as well as those traits that impact mammalian fitness. However, the punctuated resolution of microbial taxonomy may limit our ability to detect conserved gut microbes, especially in cases in which broadly related microbial lineages possess shared traits that drive their apparent ubiquity across mammals. To advance the discovery of conserved mammalian gut microbes, we developed a novel ecophylogenetic approach to taxonomy that groups microbes into taxonomic units based on their shared ancestry and their common distribution across mammals. Applying this approach to previously generated gut microbiome data uncovered monophyletic clades of gut bacteria that are conserved across mammals. It also resolved microbial clades exclusive to and conserved among particular mammalian lineages. Conserved clades often manifest phylogenetic patterns, such as cophylogeny with their host, that indicate that they are subject to selective processes, such as host filtering. Moreover, this analysis identified variation in the rate at which mammals acquire or lose conserved microbial clades and resolved a human-accelerated loss of conserved clades. Collectively, the data from this study reveal mammalian gut microbiota that possess traits linked to mammalian phylogeny, point to the existence of a core set of microbes that comprise the mammalian gut microbiome, and clarify potential evolutionary or ecologic mechanisms driving the gut microbiome’s diversification throughout mammalian evolution.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Beyond the Venn diagram: the hunt for a core microbiome.
- Record: found
- Abstract: found
- Article: not found