- Record: found
- Abstract: found
- Article: found
Effects of the small-molecule inhibitor of integrin α4, TBC3486, on pre-B-ALL cells
letter
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The treatment of patients with chemotherapy-resistant leukemia remains a challenge.
A role of the microenvironment for drug resistance of leukemia cells has been proposed.
1
We have identified the adhesion molecule integrin α4 as a central mediator of drug
resistance of pre-B-cell acute lymphoblastic leukemia (ALL).
2
We thus demonstrated that chemotherapy-resistant pre-B-ALL cells can be eradicated
in a xenograft model by concurrent blockade of α4 using natalizumab, a humanized anti-α4
antibody in clinical use against multiple sclerosis,
3
and Crohn's Disease.
2
Here, we extended our studies to an alternative α4 inhibitor, the non-peptidic small
molecule TBC3486. Previous in vitro assays and molecular modeling studies indicated
that TBC3486 behaves as a ligand mimetic, competing with VCAM-1 for the MIDAS site
of integrin α4.
4
As such, the compound has shown efficacy in integrin α4-dependent models of inflammatory
and autoimmune disease
4
and has shown efficacy in mice with autoimmune encephalomyelitis, a model for multiple
sclerosis.
5
As opposed to natalizumab, which will inhibit both members of the α4 integrin family,
α4β1 and α4β7, TBC3486 is 200-fold more potent in inhibiting α4β1 than α4β7. In addition,
it is completely inactive against all other integrins tested, including members of
the β2, β3 as well as other members of the β1 family of integrins.
4
The potential usefulness of this novel inhibitor for pre-B-ALL treatment was tested
in our established in vitro and in vivo assays.
2, 4
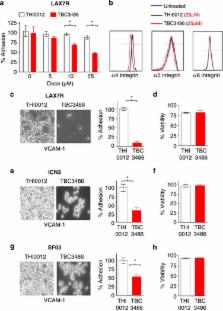
We evaluated the effect of TBC3486 on de-adhesion of patient-derived ALL cells (LAX7R)
using established adhesion assays. As a control for our studies, a close structural
analog was used that lacks activity toward α4β1 integrin (THI0012). After activating
LAX7R cells with 1 mM Mn2+, leukemia cells were co-cultured with the murine stromal
cell line OP9.
6, 7
Subsequently, LAX7R cells were treated with different doses of TBC3486 (5, 10 and
25 μM) and its control, THI0012 (5, 10 and 25 μM), for 4 days. TBC3486 dose-dependently
inhibited adhesion of ALL cells (Figure 1a), albeit the adhesion was not completely
blocked. The dose of 25 μM was selected for subsequent studies. The concentrations
of compound required for inhibition in these assays are higher than previously reported.
4
This is due to the fact that TB3486 is highly protein bound in the presence of 20%
serum (used in these assays), which significantly reduces the amount of free compound
available to bind to the integrin receptor. Next, we determined whether TBC3486 decreases
binding of three xenograft cells derived from primary pre-B-ALL cases (LAX7R, ICN3
and SFO3) to the counter-receptor of α4 integrin, human VCAM-1. Adhesion assays were
performed as previously described
4, 8, 9
by culturing ALL samples treated with TBC3486 (25 μM) or THI0012 (25 μM) on hVCAM-1-coated
plates for 2 days. Compared with the control group, TBC3486-treated ALL cells showed
significantly less adhesion to hVCAM-1 (Figures 1c, e and g); however, the adhesion
was not completely blocked. CD49d (MFI) is expressed with higher intensity in LAX7R
compared with the other two samples (ICN3 and SFO3) (data not shown), which may explain
why TBC3486 blocked a larger percentage of LAX7R adhesion to VCAM-1. In addition to
blocking cell adhesion, TBC3486 treatment also specifically targeted the expression
of integrin α4, but not integrin α5 and α6 (Figure 1b). The treatment with TBC3486
did not affect cell viability in all three cases (Figures 1d, f and h) compared with
the THI0012 control. Taken together, TBC3486 leads to the partial de-adhesion of pre-B-ALL
cells from its counter-receptor VCAM-1 under the conditions described.
We previously showed that antibody-mediated integrin α4 blockade can sensitize leukemia
cells to chemotherapy.
2
Therefore, we evaluated whether TBC3486 can not only de-adhere cells from its counter-receptors
but also sensitize them to chemotherapy. To study the drug sensitivity of human leukemia,
ALL (LAX7R) cells were co-cultured with murine calvaria-derived OP9 stromal cells,
which allow in vitro studies beyond 2 days. Then this co-culture was exposed to chemotherapy
as previously described.
9, 10
ALL cells were treated with chemotherapy (vincristine, dexamethasone, L-asparaginase;
VDL) or saline as control for 4 days, with TBC3486 (25 μM) or control THI0012. TBC3486
with VDL treatment reduced the cell viability of human leukemia LAX7R (51±1% vs 31±1%
P<0.05) (Figure 2a), ICN3 (60±3% vs 49±1% P<0.05) (Figure 2b) and SFO3 (78±1% vs 65±1%
P<0.05) (Figure 2c), indicating that this combination therapy sensitized leukemia
cells to chemotherapy in vitro. Nevertheless, all chemoprotection afforded by OP9
cells was removed by TBC3486 in spite of only partial de-adhesion. This observation
at least suggests that effects over and above adhesion and physical chemoprotection
provided by stromal cells are mediated through α4 integrin.
To determine whether TBC3486 can prolong the survival of xenotolerant mice bearing
human leukemia cells in vivo, LAX7R cells were injected into NOD/SCID hosts. Three
days after leukemia cell transfer, mice received either TBC3486 or THI0012 (control)
(10 mg/kg/d) daily for 2 weeks with two daily intraperitoneal injections to ensure
stable plasma levels, with or without VDL chemotherapy. TBC3486-treatment (n=7) resulted
in prolonged survival time compared with THI0012 control mice (n=3) (median survival
time (MST)=41 vs 33 days, P=0.001). In combination with chemotherapy, the Kaplan–Meier
survival curve indicated that TBC3486+VDL-treatment (n=6) resulted in a significantly
prolonged survival time (compared with the VDL+THI0012 control (n=7) (MST=82 vs 58
days, P=0.0003; Figure 2d).
TBC3486 preferentially inhibits the high-affinity form of the integrin α4.
4
It may thus interfere with the function of lymphocytes entertaining the inflammatory
response in multiple sclerosis, but may not interfere with that of lymphocytes causing
inflammatory bowel disease. Which β-integrin is the more relevant partner for α4 in
the context of drug resistance in ALL is unclear. In addition, TBC3486 mimics VCAM-1
binding, inducing the high-affinity conformation of integrin α4; by contrast, natalizumab
recognizes and blocks the ligand-binding moiety of α4 in any conformation and without
affecting activation status. Whether, therefore, TBC3486-binding of integrin α4 might
elicit outside-in signaling would have to be addressed in further studies. Although
unknown off-target effects of the compound cannot be excluded, it should be emphasized
that the control compound, THI0012, is structurally identical to TBC3486 except it
is the opposite enantiomer. This simple change in stereochemistry eliminates its activity
toward α4β1. The fact that this control compound has no activity in any of the in
vitro or in vivo studies described here also argues that the efficacy of TBC3486 in
these studies is directly related to its activity toward α4β1 integrin.
Like many small molecules, TBC3486 is endowed with a rather shorter half-life, requiring
more frequent dosing than natalizumab. A shorter half-life should likely be an advantage
in leukemia treatment, but challenges at establishing the optimal timing of anti-integrin
treatments (how often, how much and so on) relative to the chemotherapy must not be
underestimated. Natalizumab led to the complete eradication of pre-B-ALL in our xenotransplant
model in two out of three pre-B-ALL cases. By contrast, adjuvant treatment with TBC3486
was associated only with prolonged survival, not with leukemia cell eradication. Although
formal side-by-side comparisons were not performed, this may at least in part be due
to the differences in treatment schedules (TBC3486: twice daily for 2 weeks, natalizumab:
once weekly for 4 weeks), but additional differences of the two compounds may also
contribute. As the half-life of natalizumab is much longer, therapeutic drug levels
are likely to be continuously maintained for the entire duration of the 4-week VDL
cycle.
Taken together, our data demonstrate that small-molecule inhibition of integrin α4
using TBC3486, currently in preclinical evaluation, is a promising approach for targeting
chemotherapy-resistant leukemia. Further studies are warranted to understand and evaluate
preclinically adjuvant small-molecule inhibition of integrins to overcome relapse
of ALL.
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Leukemia stem cells and microenvironment: biology and therapeutic targeting.
T. Jordan, M Konopleva (2011)
- Record: found
- Abstract: found
- Article: not found
Small molecule inhibition of CBP/catenin interactions eliminates drug resistant clones in acute lymphoblastic leukemia
Eun Gang, Yao-Te Hsieh, Jennifer Pham … (2013)
- Record: found
- Abstract: found
- Article: not found
Integrin alpha4 blockade sensitizes drug resistant pre-B acute lymphoblastic leukemia to chemotherapy.
Eugene Park, Sandra Huantes, Reshmi Parameswaran … (2013)