- Record: found
- Abstract: found
- Article: found
Incidence of pregnancy loss and characterization of fetal development in red pandas
Abstract
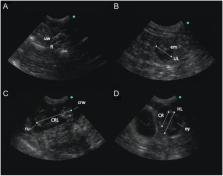
Previous reports indicate that red pandas ( Ailurus fulgens styani) may experience fetal loss during gestation; however, neither the rate nor timing of pregnancy failure has been described in this species. The objective of this study was to utilize ultrasound video and images collected between 2010 and 2020 at the Cincinnati Zoo and Botanical Garden to better characterize pregnancy loss and fetal development. Trans-abdominal ultrasound examinations were performed on six female red pandas over a 10-year period, resulting in 12 profiles. Pregnancy was diagnosed via ultrasound in 10 of 12 profiles, and 40.0% of pregnancies showed evidence of fetal loss prior to parturition. Pregnancy loss was classified into lost (2 of 10; 20.0%), in which no cubs were produced, or partial loss (2 of 10; 20.0%), in which two concepti were visualized via ultrasound, but only one cub was born. Fetal loss occurred between days 51 and 23 pre-partum. Fetal growth characteristics were documented, including skeletal ossification (occurring between days 32 and 27 pre-partum), crown-rump length, head length, cranial length, and fetal heart rate (173–206 b.p.m.). These findings provide novel insights into pregnancy loss, may serve as a reference for milestones of fetal development, and may be useful in diagnosing pregnancy and assessing pregnancy loss in red pandas.
Lay summary
For many wildlife species, there is no non-invasive method of determining pregnancy; therefore, the rate of pregnancy loss oftentimes is unknown. Many red pandas in human care that are paired for breeding are observed exhibiting normal mating behaviors; however, only a relatively low proportion of females produce cubs. We utilized animals conditioned for ultrasound examination to diagnose pregnancy and characterize the incidence and timing of pregnancy loss. In total, 12 potential pregnancies were monitored, beginning after breeding season and ending ~2 weeks prior to anticipated cubbing. Of these, ten were (83.3%) were diagnosed as pregnant, with 40% undergoing either full or partial pregnancy loss. Fetal growth characteristics, such as body length and head size, are described which may be useful for monitoring pregnancies and estimating fetal age. Results of this study provide novel data on pregnancy loss in red pandas. Insights into the rate and timing of reproductive failure may illuminate causes and contributing factors, ultimately allowing for improvements in husbandry which may result in greater reproductive success of individuals recommended for breeding.
Related collections
Most cited references24

- Record: found
- Abstract: found
- Article: found
Genomic evidence for two phylogenetic species and long-term population bottlenecks in red pandas
- Record: found
- Abstract: found
- Article: not found
Canine embryonic and fetal development: a review.

- Record: found
- Abstract: found
- Article: found
 This work is licensed under a
This work is licensed under a