- Record: found
- Abstract: found
- Article: not found
Introduction to Food Irradiation and Medical Sterilization
chapter-article
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
This chapter is comprehensive overview of sterilization and disinfectant processes
used for food-borne disease control and medical sterilization. Pathogens such as bacteria,
viruses, and endospores are described along with other infectious agents. The processes
for controlling these infectious agents in food are summarized. These processes include
not only irradiation by the two most important processes, electron beam and gamma
ray, but by other processes such as ultraviolet, microwave, and infrared radiation.
Medical sterilization and disinfectant processes are reviewed. Besides irradiative
processes, thermal processes such as steam autoclave and dry heat are reviewed. Many
liquid and gaseous chemical disinfectants are covered. The commercially important
ethylene oxide sterilization process is discussed. Dealing with bioterrorism agents
is briefly discussed. Throughout this chapter, the mechanisms, the irradiation, sterilization,
and chemical disinfectant processes used to destroy the pathogens are discussed often
in chemical detail.
This first chapter primarily focuses on the processes used to destroy microbiological
life. Microbiological life, microbes, or pathogens in food contribute to food spoilage,
disease, illness, and even death. Similarly, microbes in a health care environment
may cause disease, infections, sickness, illness, and death. The elimination, killing,
or control of microbes is therefore critically important. The processes used for microbe
control can affect the properties of materials, especially plastics. That effect is
the subject of the bulk of this book. This introductory chapter will be split up into
three parts. First will be background on the biology and types of microbes. Then the
processes used to control microbial life in the food supply are discussed. That is
followed by the control of microbes in the health care environment.
1.1
Pathogens
Sterilization processes are intended to deal with contamination of food, surfaces,
or equipment by potentially harmful life forms. These life forms are primarily bacteria,
viruses, and endospores, but could also include parasites, prions, molds, fungi, yeasts,
protozoa, etc. Organisms that cause disease are called pathogens. This section briefly
summarizes what these life forms are, which leads to a better understanding of how
sterilization processes kill or control them.
1.1.1
Bacteria
Bacteria consist of only a single cell. Bacteria fall into a category of life called
the prokaryotes. Prokaryotes' genetic material, or DNA, is not enclosed in a cellular
compartment called a nucleus as it is in eukaryotes. Not all bacteria are harmful.
However, the subject of this book implies the killing of harmful bacteria. Most people
know harmful bacteria best because of the diseases they cause. Some of these diseases
are produced when bacteria attack directly the tissues of a plant or animal. For example,
fruits and vegetables that become discolored as they are growing may be under attack
by bacteria, though discoloration may also be the result of oxidation.
Bacteria also attack cells by releasing chemicals that are poisonous to plants and
animals. Such poisons are known as toxins. As an example, Clostridium botulinum releases
a toxin that causes the most severe form of food poisoning, botulism.
Some forms of dangerous bacteria live on the human skin, but cause no harm unless
they are able to enter the body. Among these bacteria is Staphylococcus, responsible
for the potentially fatal toxic shock syndrome and gastroenteritis. The bacteria may
enter the bloodstream through a break in the skin or the digestive tract through contaminated
food. And although Escherichia coli is helpful within the digestive system, if it
is ingested it may cause cramping and diarrhea. If it enters the bloodstream, it causes
fever, hypotension, altered mental status, and possibly death.
A diagram of a typical bacterium is shown in Fig. 1.1
and a micrograph of cholera bacteria is shown in Fig. 1.2
. Bacteria can be of many other shapes, however, some of which are shown in Fig. 1.3
.
Figure 1.1
Diagram of a typical bacterium.
1
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.2
Scanning electron micrograph of cholera bacteria.
1
Figure 1.3
Common bacterial morphologies.
Bacteria are often classified as Gram positive and Gram negative. The main difference
between the two forms of bacteria is the composition and structure of the cell walls
of the two, as shown in Fig. 1.4
. Referring to the figure:
•
Peptidoglycan, also known as murein, is a polymer consisting of sugars and amino acids
that forms a mesh-like layer outside the plasma membrane of bacteria forming the cell
wall.
•
The periplasm is a space bordered by the two selective permeable biological membranes,
which are the inner plasma membrane and the outer membrane in Gram-negative bacteria.
•
There is no periplasmic space in Gram-positive bacteria because there is only one
biological membrane, but a region called inner wall zone has been observed between
the cytoplasmic membrane and the bacterial cell wall.
Figure 1.4
The structural difference of the bacterial cell walls of Gram-positive and Gram-negative
bacteria.
1
For color version of this figure, the reader is referred to the online version of
this book.
The two types of bacteria may respond differently to sterilization processes. Sterilization
processes that kill bacteria usually do so by:
•
Causing damage to the physical structure, particularly rupture of the cell walls
•
Altering the membrane permeability
•
Damaging the proteins within the cells
•
Damaging the nucleic acids in the DNA or RNA.
1.1.2
Viruses
A virus is a small infectious agent that can replicate only inside the living cells
of organisms. They are too small to be seen with a light microscope. Viruses are not
plants, animals, or bacteria, but they are more like parasites. They are parasites
because without a host cell, they cannot carry out their life-sustaining functions
or reproduce. Although they may seem like living organisms because of their reproductive
abilities, viruses are not living.
The average virus is about one-hundredth the size of the average bacterium. All viruses
contain nucleic acid, either DNA or RNA (but not both), and a protein coat, which
encases the nucleic acid. Viruses come in a wide diversity of shapes and sizes called
morphologies.
2
Viruses are inactive when outside of a living cell, but their nucleic acid once entering
the cell can take over the cells' activities, which is primarily to reproduce and
break out of the cell to infect other cells. Figure 1.5
shows electron micrographs of two different viruses. The micrograph of the virus on
the right side clearly shows the DNA and cell wall structure.
Figure 1.5
Electron micrographs of two viruses, a bacteriophage on the left and an influenza
virus on the right.
3
For color version of this figure, the reader is referred to the online version of
this book.
1.1.3
Endospores
An endospore is a dormant, tough, and temporarily nonreproductive structure produced
by certain bacteria. It is a stripped-down, dormant form to which the bacterium can
reduce itself when conditions are not right for the bacterium to thrive. It is very
resistant to harsh environments and can remain viable for very long periods of time,
only to spring back to life when conditions are right.
The structure and a micrograph of an endospore are shown in Fig. 1.6
. The endospore consists of the bacterium's DNA and part of its cytoplasm, surrounded
by a very tough outer coating. The coating consists of multiple layers. The outermost
layer is the exosporium, which is a thin protein covering. The next layer is the spore
coat which is comprised of spore-specific proteins. An outer membrane surrounds the
cortex. The cortex is composed of peptidoglycan containing some dipicolinc acid and
calcium ions that cross-link. This creates a highly impenetrable layer that provides
the heat resistance properties of the endospore. The core contains the cell wall and
cytoplasmic membrane, nuclear material, some ribosomes, RNA molecules, and enzymes.
The core contains much less water than the bacterial cell.
Figure 1.6
Structure of an endospore on the left and a micrograph of anthrax spores on the right.
For color version of this figure, the reader is referred to the online version of
this book.
Endospores are highly resistant to hostile physical and chemical conditions. They
allow a bacterium to survive suboptimal environmental conditions. Because these spores
are resistant to heat, radiation, disinfectants, and desiccation, they are difficult
to eliminate (but not impossible) from medical and pharmaceutical materials and are
a frequent cause of contamination.
1.1.4
Other Infectious Agents
There are other infectious agents may require control. These are listed in the next
few sections.
1.1.4.1
Prions
A prion is an infectious agent, rather than a life form, composed of protein in a
misfolded form. The group of fatal neurodegenerative diseases are called transmissible
spongiform encephalopathies that include bovine spongiform encephalopathy (also known
as “mad cow disease”) in cattle and Creutzfeldt–Jakob disease (CJD) in humans. They
occur in animals (dogs, cows, and primates) as well as humans and are rapidly fatal
once symptoms develop. Prions propagate by transmitting a misfolded protein state.
When a prion enters a healthy organism, it induces existing, properly folded proteins
to convert into the disease-associated, prion form. It acts as a template to guide
the misfolding of more protein into prion form. This altered structure is extremely
stable and accumulates in infected tissue, causing tissue damage and cell death. CJD
poses a unique infection prevention problem because prions, which are protein-containing
infectious agents, can survive many common sterilization processes.
1.1.4.2
Helminthes
A parasitical organism is a life form that lives off the host. Helminthes or parasitic
worms are a division of eukaryotic parasites. They are worm-like organisms that live
and feed off living hosts, receiving nourishment and protection while disrupting their
hosts' nutrient absorption, causing weakness and disease. Those that live inside the
digestive tract are called intestinal parasites. Figure 1.7
shows one of these parasitic worms. They can live inside humans as well as other animals.
The helminthes not only reside in the digestive tract. They may reside in other tissues.
Trichinosis is a well-known example. Trichinosis is infection with the microscopic
roundworm Trichinella spiralis. A micrograph of the trichinosis parasites in muscle
tissue is shown in Fig. 1.8
.
Figure 1.7
Micrograph of hookworm parasites on intestinal wall.
1
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.8
Micrograph of trichinosis parasites in muscle tissue.
1
For color version of this figure, the reader is referred to the online version of
this book.
1.1.4.3
Fungi: Molds and Yeasts
A fungus is a eukaryotic organism. Fungal cells have cell walls that contain chitin,
unlike the cell walls of plants, which contain cellulose. Chitin is a polymer of N-acetylglucosamine.
Molds are fungi that grow in the form of multicellular filaments called hyphae, such
as shown in Fig. 1.9
, which shows other labeled features. Microscopic fungi that grow as single cells
are called yeasts. Figure 1.10
shows a micrograph of a yeast.
Figure 1.9
Micrograph of Penicillium showing (1) hypha, (2) conidiophore, (3) philalide, (4)
conidia, and (5) septa.
1
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.10
Micrograph of yeast species Saccharomyces cerevisiae.
1
Some molds cause disease or food spoilage. Many fungi produce biologically active
compounds, several of which are toxic to animals or plants and are therefore called
mycotoxins. Of particular relevance to humans are mycotoxins produced by molds causing
food spoilage. Inhaled fungal spores are a well-known cause of allergy and asthma.
Some human diseases caused by fungi include:
•
Aspergillosis
•
Candidiasis
•
Coccidioidomycosis
•
Histoplasmosis
•
Mucormycosis
•
Opportunistic pneumonia
•
Thrush.
As such, there is a need to control or kill fungi in the food-processing and in the
medical environment.
1.1.4.4
Protozoans
Protozoa are unicellular eukaryotes, meaning that they have characteristic organelles.
They are relatively large and some are visible with the naked eye. Not all protozoa
are of concern to public health.
Those that are pathogens include:
•
Toxoplasma
•
Isospora
•
Cryptosporidium
•
Cyclospora
•
Sarcocystis
•
Plasmodium
•
Giardia
•
Cryptobia species
•
Enterocytozoon bieneusi
•
Pleistophora species.
However, there have been rare infections by amoeba, Naegleria fowleri, in the news
recently. The news media have called these “brain-eating amoeba.” For the infections
in the news, the amoeba entered the brain after contaminated warm water entered the
nose, though there were also cases caused by head trauma.
1.1.5
Biofilm
A biofilm is an aggregate of microorganisms in which cells adhere to each other on
a surface. Dental plaque, the slimy coating that fouls pipes and tanks, and algal
mats on bodies of water are examples of biofilms. Biofilms can form on solid or liquid
surfaces as well as on soft tissue in living organisms. They are typically resistant
to conventional methods of disinfection. These adherent cells in a biofilm are frequently
embedded within a self-produced matrix of extracellular polymeric substance (EPS).
Biofilm EPS, which is also referred to as slime (although not everything described
as slime is a biofilm), is a polymeric conglomeration generally composed of extracellular
DNA, proteins, and polysaccharides. Biofilms may form on living or nonliving surfaces
and can be prevalent in natural, industrial, and hospital settings.
Biofilms have been found to be involved in a wide variety of microbial infections
in the body. Biofilms have been implicated in common problems such as urinary tract
infections, catheter infections, middle-ear infections, formation of dental plaque,
gingivitis, and coating contact lenses. Though less common but more lethal, biofilms
have also been implicated in health problems such as endocarditis, infections in cystic
fibrosis, and infections of permanent implanted devices such as joint prostheses and
heart valves.
1.2
Food-Borne Disease Control
Concern over food-borne diseases is high. These are the illnesses contracted from
eating contaminated foods or beverages. These illnesses include food-borne intoxications
and infections and are often incorrectly referred to as food poisoning. There are
more than 250 different food-borne diseases and they are caused by viruses, bacteria,
parasites, toxins, metals, and prions. According to the US Center for Disease Control
(CDC), 31 pathogens are known to cause 20% of the food-borne illnesses, with 80% being
caused by unknown agents. Studies by the CDC in 2010 estimated that food-borne diseases
each year cause roughly one in six Americans (or 48 million people) to get sick; 128,000
are hospitalized, and 3000 die of food-borne diseases. Symptoms of food-borne illness
range from mild gastroenteritis to life-threatening neurologic, hepatic, and renal
syndromes. Some of these illnesses are well known and include: botulism, brucellosis,
Campylobacter enteritis, E. coli, hepatitis, listeriosis, salmonellosis, shigellosis,
toxoplasmosis, viral gastroenteritis, teniasis, and trichinosis. Food-borne diseases
pose a widespread threat to human health and they may lead to reduced economic productivity.
Economic losses associated with such food-borne diseases are high estimated between
US $6500 million and $33,000 million.
Eight known pathogens account for the vast majority of illnesses, hospitalizations,
and deaths. Table 1.1, Table 1.2, Table 1.3
list the top five pathogens causing illness, hospitalization, and death.
Table 1.1
Top Five Pathogens Contributing to Domestically Acquired Food-Borne Illnesses
4
Pathogen
Estimated Number of Illnesses
90% Credible Interval
% of Total
Norovirus
5,461,731
3,227,078–8,309,480
58
Salmonella, nontyphoidal
1,027,561
644,786–1,679,667
11
Clostridium perfringens
965,958
192,316–2,483,309
10
Campylobacter species
845,024
337,031–1,611,083
9
Staphylococcus aureus
241,148
72,341–529,417
3
Subtotal
91
Table 1.2
Top Five Pathogens Contributing to Domestically Acquired Food-Borne Illnesses Resulting
in Hospitalization
4
Pathogen
Estimated Number of Hospitalizations
90% Credible Interval
% of Total
Salmonella, nontyphoidal
19,336
8545–37,490
35
Norovirus
14,663
8097–23,323
26
Campylobacter species
8463
4300–15,227
15
Toxoplasma gondii
4428
3060–7146
8
Escherichia coli (STEC) O157
2138
549–4614
4
Subtotal
88
Table 1.3
Top Five Pathogens Contributing to Domestically Acquired Food-Borne Illnesses Resulting
in Death
4
Pathogen
Estimated Number of Deaths
90% Credible Interval
% of Total
Salmonella, nontyphoidal
378
0–1011
28
Toxoplasma gondii
327
200–482
24
Listeria monocytogenes
255
0–733
19
Norovirus
149
84–237
11
Campylobacter species
76
0–332
6
Subtotal
88
Beyond the illnesses, there is the economic cost of high food losses from infestation,
contamination, and spoilage caused by insects and bacteria. Killing the microbes and
insects that cause these losses by food irradiation can help reduce these losses.
Even though there have been ways to preserve foods for centuries, if not millenia,
the cause was not understood until the mid-nineteenth century.
As mentioned in the introduction of this chapter, the killing of microbes in food
is a large-scale or bulk process. Heat is one of the best ways to kill microbes, but
it is not often desirable to cook the foods prior to sale. The irradiation processes
using gamma, electron beam (or beta), infrared (IR), and microwave are used. However,
the problem of food preservation has long been accepted and over time have ways have
been developed to preserve food. The history of food preservation is interesting.
1.2.1
The History of Food Preservation
Many primitive and tedious methods of food preservation have been used. Some of these
were developed thousands of years ago and are still used today. These include:
•
Drying – Drying was and still is used to preserve fruit, vegetables, meats, and fish.
Foods can be dried in the sun (as the ancients did), in an oven or in a food dehydrator
by using the right combination of warm temperatures, low humidity, and air current.
Drying removes the moisture from the food, so bacteria, yeast, and mold that need
water cannot grow and spoil the food.
•
Chemical pickling – Food for pickling is placed in an edible liquid that inhibits
or kills bacteria and other microorganisms. There are many pickling agents including
brine (high in salt), vinegar, alcohol, and vegetable oil (particularly olive oil).
Many chemical pickling processes also involve heating or boiling so that the food
being preserved becomes saturated with the pickling agent. In fermentation pickling,
the food itself produces the preservation agent, typically by a process that produces
lactic acid. This is commonly used to preserve vegetables.
•
Salting – Salting has been used extensively for pork, beef, and fish. The origins
of salt-preserving food can be traced back to at least ancient Egypt, where they used
salt as part of the embalming process, as well as in food preservation.
•
Sugaring – Sugaring is used to preserve fruits for the winter, producing jams and
jellies. Sugaring is the process of desiccating a food by first dehydrating it and
then packing it with pure sugar. The sugar can be crystalline table or raw sugar,
or it can be a high-sugar-density liquid such as honey, syrup, or molasses.
•
“Cold storage” – Root cellars, ground burial, and iceboxes were cold storage methods
used before the development of refrigeration.
•
Smoking – Smoking is the exposure of cured meat and fish products to smoke for the
purposes of preserving them and increasing their palatability by adding flavor and
imparting color. The drying action of the smoke tends to preserve the meat, though
many of the chemical species present in wood smoke (e.g. formaldehyde and certain
alcohols) are natural preservatives as well. Smoking is one of the oldest of food
preservation methods.
•
Canning – Canning is a method of preserving food in which the food contents are processed
and sealed in an airtight container. Canning was developed around 1810 by Nicolas
Appert in Paris. It revolutionized food preservation.
•
Blanching – Also known as parboiling, blanching is the method by which foods are partially
cooked as a way to preserve their color, texture, and flavor.
1.2.2
The History of Food Irradiation
Antoine-Henri Becquerel (1852–1908) is known for his discovery of radioactivity in
1895, for which he received the 1903 Nobel Prize for Physics jointly with Marie Curie
and Pierre Curie. X-rays were also discovered in 1895 by W.K. Roentgen. Ionizing radiation
was found to be lethal to living organisms soon after these discoveries. The idea
of using ionizing radiation in food preservation almost immediately followed. The
suggestion to use ionizing energy to destroy pathogenic and spoilage microorganisms
in food was published in a German medical journal the same year. However, the cost
and quantity of this radiation inhibited commercial development until much, much later.
The basic history of food irradiation follows
5
:
•
1905 – Scientists receive patents for a food preservative process that uses ionizing
radiation to kill bacteria in food.
•
1921 – US patent is granted for a process to kill Trichinella spiral in meat by using
X-ray technology.
•
1953–1980 – The US Government forms the National Food Irradiation Program. Under this
program, the US Army and the Atomic Energy Commission sponsor many research projects
on food irradiation. The US Army's interest is feeding soldiers during wartime.
•
1958 – The Food, Drug, and Cosmetic Act is amended and defines sources of radiation
intended for use in processing food as a new food additive. The Act is administered
by Food and Drug Administration (FDA).
•
1963 – FDA approves irradiation to control insects in wheat and flour.
•
1964 – FDA approves irradiation to inhibit sprouting in white potatoes.
•
1964–1968 – The US Army and the Atomic Energy Commission petition FDA to approve the
irradiation of several packaging materials.
•
1966 – The US Army and US Department of Agriculture (USDA) petition FDA to approve
the irradiation of ham.
•
1971 – FDA approves the irradiation of several packaging materials based in the 1964–68
petitions by the US Army and the Atomic Energy Commission.
•
1976 – The US Army contracts with commercial companies to study the wholesomeness
of irradiated ham, pork, and chicken.
•
1980 – USDA inherits the US Army's food irradiation program.
•
1985 – FDA approves irradiation at specific doses to control Trichinella spiral is
in pork. FDA approves irradiation at specific doses to delay maturation, inhibit growth,
and disinfect foods, including vegetables and spices. The Federal Meat Inspection
Act is amended to permit gamma radiation to control Trichinella spiral is in fresh
or previously frozen pork. Law is administered by USDA.
•
1990 – FDA approves irradiation for poultry to control Salmonella and other food-borne
bacteria.
•
1992 – USDA approves irradiation for poultry to control Salmonella and other food-borne
bacteria.
•
1997 – FDA's regulations are amended to permit ionizing radiation to treat refrigerated
or frozen uncooked meat, meat by-products, and certain food products to control food-borne
pathogens and to extend shelf life.
•
2000 – USDA's regulations are amended to allow the irradiation of refrigerated and
frozen uncooked meat, meat by-products, and certain other meat food products to reduce
the levels of food-borne pathogens and to extend shelf life. FDA's regulations are
amended to permit the irradiation of fresh shell eggs to control Salmonella.
1.2.3
Dosage Measures of Radiation
The radiation dose for food irradiation and sterilization generally is measured using
the conventional unit rad or the SI unit gray (Gy). The rad, which stands for radiation
absorbed dose, was the conventional unit of measurement, but it has recently been
replaced by the Gy; 1 Gy is equal to 100 rad. The prefix kilo (k), for 1000×, is commonly
used with the gray unit, kGy. Mega (M) for 1,000,000× is often used with the rad unit
(Mrad). This is summarized in Table 1.4
.
Table 1.4
Conversion of Common Rad and Gray Units of Irradiation
The Rad (rad) is Replaced by the Gray (Gy)
The Gray (Gy) Replaces the Rad (rad)
1 megarad (Mrad) = 10 kilogray (kGy)
1 kilogray (kGy) = 0.1 (Mrad)
1 kilorad (krad) = 10 gray (Gy)
1 gray (Gy) = 100 rad (rad)
1 rad (rad) = 10 milligray (mGy)
1 milligray (mGy) = 100 millirad (mrad)
1 millirad (mrad) = 10 microgray (μGy)
1 microgray (μGy) = 100 microrad (μrad)
1 microrad (μrad) = 10 nanogray (nGy)
1 nanogray (nGy) = 100 nanorad (nrad)
There are other radiation measures: Curie (Ci), Becquerel (Bq), Roentgen (R), Coulomb/kg
(C/kg), The rem (rem), and Sievert (Sv). However, these are used for other purposes.
1.2.4
Electron Beam Irradiator
Electron beam irradiation is sometimes referred to as beta irradiation. Beta particles
are high-energy, high-speed electrons or positrons emitted by certain types of radioactive
nuclei such as potassium-40. Beta particles are subatomic particles ejected from the
nucleus of some radioactive atoms. They are equivalent to electrons. The difference
is that beta particles originate in the nucleus and electrons originate outside the
nucleus.
Electron beam radiation breaks the DNA or damages other critical molecules in bacteria,
either killing them or preventing them from reproducing.
The procedure for electron beam irradiation is relatively simple. In practice, a continuous
process is used to improve the economics of the process. The source of electron beams
is an “accelerator.” The accelerator converts electricity through a tungsten filament
source.
Accelerators generate and accelerate electrons and direct them toward the food product
needing irradiation. An accelerator (see Fig. 1.11
) consists of four major systems:
1.
Voltage generator
2.
Acceleration tube and electron gun
3.
Scan chamber and scan horn
4.
A control system.
Figure 1.11
Diagram of an electron beam generator.
Each segment plays a key role and is briefly described. Low voltage power, typically
three-phase, 440 V AC, is converted to high-frequency (100 kHz) radio frequency (RF)
power by an oscillator. A number of rectifiers in series along with an RF resonant
transformer convert the RF power into ultra high voltage (2–5 MM V) direct current
(DC) power. The DC voltage is sent to the acceleration tube. This tube is a hollow
vacuum cylinder made from glass and metal. Glass rings are positioned as insulation
rings in between metal rings, called dynodes. An electron gun is positioned at the
entry point of the tube. A tungsten filament is the source of electrons that are emitted
by heating the filament. The continuous electron stream is accelerated through a vacuum
tube, supplying a high-energy beam with a diameter of 2–3 cm. The accelerated beam
is transmitted through an oscillating magnetic field with a frequency of 200 Hz that
converts the tight beam cone into a wide curtain of electrons with a width of 1–2.5 m.
A thin titanium window acts as a window or a filter before the beam reaches the food
to be irradiated. A control system monitors all the functions of the various systems
of accelerator to insure proper and consistent operation.
An accelerator operates based on a number of factors including voltage (electron energy),
beam current, and beam power. Because electrons have mass, they can only penetrate
about 1.5 inches (3.8 cm) into a typical food product or about 3.5 inches (8.9 cm)
if the food product is irradiated on both sides. Electrons also have an electric charge.
This charge allows the stream of accelerated electrons to be scanned by magnets to
track across the product. A commercial food electron beam irradiator accelerates the
electrons to an energy of up to 10,000,000 electron volts [10 million electron volts
(MeV)]. Electron beam irradiators typically use massive concrete, steel, or lead shielding.
Electron beam accelerators can be turned on and off. Safety interlocks ensure that
a person cannot enter the radiation chamber where the food is being irradiated when
the accelerator is “on.” Product is usually passed through the scanned “beam” on roller-type
conveyors as shown in Fig. 1.12
. A picture of the electron beam scan horn and conveyor system in the irradiation
area is shown in Fig. 1.13
.
Figure 1.12
A schematic of an electron beam irradiation facility with two-side irradiation for
food irradiation. For color version of this figure, the reader is referred to the
online version of this book.
Figure 1.13
Scan horn and conveyor system of an Iotron IMPELA® 10/50 Linear Electron Accelerator
Installed at Iotron Industries USA Inc., Columbia City, Indiana (photo courtesy of
Iotron Industries Canada/USA Inc.). For color version of this figure, the reader is
referred to the online version of this book.
1.2.5
Gamma Irradiator
Gamma radiation, signified by the Greek letter γ, is one of the three types of natural
radioactivity. Gamma rays are electromagnetic radiation, like X-rays, except even
more energetic. They have enough energy to ionize matter and can damage or destroy
living cells, which is of course why they are useful for sterilization. The source
of gamma rays in a gamma irradiator is Cobalt-60. Cobalt-60 radioactively beta decays
to Nickel-60 and emits two gamma rays with energies of 1.17 and 1.33 MeV.
6
Cobalt-60 is not found in nature. It is a synthetic radioactive isotope made by neutron
activation of Cobalt-59. Cobalt-60 is produced off site in nuclear reactors and transported
in special shipping containers (casks) to the sterilization facility. Cobalt-60 is
a solid radioactive metal molded into a cylindrical slug as shown in Fig. 1.14
. The slugs are loaded into “sealed source” pencils made of stainless steel. The stainless
steel sealed source contains the “radioactive” Cobalt-60, but allows the gamma photons
(radiation) to pass through the steel. The pencils are arranged into flat panel arrays.
Cobalt-60 continuously emits radiation and cannot be turned “off.” The arrays are
stored in a deep water pool, which prevents gamma radiation from escaping. The shielding
water does not become radioactive.
Figure 1.14
Cobalt-60 slugs are arranged into source racks [images/photos courtesy of Nordion
(Canada) Inc.]. For color version of this figure, the reader is referred to the online
version of this book.
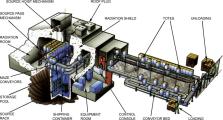
Large-scale gamma irradiators come in two configurations. In one configuration, the
Cobalt arrays are hoisted out of the water into a radiation chamber that typically
has shields made out of massive concrete or steel. This is shown in Fig. 1.15
. The concrete walls are typically 2-feet thick or more. When the irradiators are
out of the protective storage pool, all workers are outside the thick walls. Hanging
carriers, totes, or roller conveyors are typically employed to move the product through
the chamber for irradiation. A typical product container can be 60 cm × 50 cm × 150 cm,
and some irradiators are designed to irradiate entire pallets of product measuring
120 cm × 100 cm × 150 cm. Because Cobalt-60 photons have no mass, they can penetrate
more than 24 inches (60 cm) of food product if irradiated on both sides.
Figure 1.15
A schematic of a gamma irradiation facility [images/photos courtesy of Nordion (Canada)
Inc.]. For color version of this figure, the reader is referred to the online version
of this book.
In the second configuration, unlike a radiation chamber irradiator, an underwater
irradiator stores the Cobalt-60 permanently at the bottom of a pool of water. Instead
of raising the Cobalt-60 into a shielded chamber, the product, placed in water-free
containers, is lowered to the bottom of the pool adjacent to the Cobalt-60 to receive
a dose of radiation. No above-ground shielding or radiation chamber is present. Typically,
the product is loaded into water-free containers and the containers are lowered/raised
using a hoist mechanism.
1.2.6
X-Ray Irradiator
X-rays are photons and have similar properties to gamma rays emitted by Cobalt-60.
However, X-rays are generated by using an electron beam accelerator and converting
the electron beam (up to 7.5 MeV) to photons by accelerating the electrons into a
high-density material such as tungsten, steel, or tantalum. The sudden deceleration
of the electrons generates X-rays and waste heat. The shielding and product conveyance
are similar to that of a chamber-type gamma irradiator (Fig. 1.15). The advantages
of X-rays over electron beams are that they have good product penetration (over 24
inches or 60 cm of food product if irradiated on both sides). The advantage of X-rays
over gamma irradiators is that they do not require a shielding storage pool. However,
there is a substantial loss of energy during the conversion process. Thus, it suffers
a severe cost disadvantage when compared to other types of irradiators for the same
product volume throughput. For food irradiation, X-ray machines can have a maximum
energy of 5 MeV.
1.2.7
Ultraviolet Germicidal Irradiation
Ultraviolet (UV) is the part of the electromagnetic spectrum between visible light
and X-rays. The UV spectrum can be broken down into four parts as shown in Table 1.5
. The specific portion of the UV spectrum between 185 and 400 nm (also known as UV-C)
has a strong germicidal effect, with peak effectiveness at 265 nm. At these wavelengths,
UV kills microorganisms by penetrating their cell membranes and damaging the DNA or
RNA, making them unable to reproduce and effectively killing them.
Table 1.5
Four Types of Ultraviolet (UV) Light
Wavelength Range
Description
400–315 nm
UV-A – black light UV
315–280 nm
UV-B – dangerous UV
280–200 nm
UV-C – germicidal UV at 254 nm
200–100 nm
UV-V – vacuum UV
Ultraviolet germicidal irradiation (UVGI) is a sterilization method that uses UV light.
It is used in a variety of applications, such as food, air, and water purification.
It is effective in destroying the nucleic acids in these organisms so that their DNA
is disrupted by the UV radiation. This removes their reproductive capabilities and
kills them.
The application of UVGI to sterilization has been an accepted practice since the mid-twentieth
century. It has been used primarily in medical sanitation and sterile work facilities.
Figure 1.16
shows the use of UV irradiation in a laboratory. UVGI technology is particularly suited
to the beverage, bottled water, and food processing sectors. The design of these systems
is simple as shown in Fig. 1.17
. UVGI disinfection has many advantages over alternative methods. Unlike chemical
biocides, UVGI does not introduce toxins or residues into the process (which themselves
have to be subsequently removed) and does not alter the chemical composition, taste,
odor, or pH of the product.
Figure 1.16
Ultraviolet irradiation in use in a laboratory.
1
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.17
The flow-through design of a ultraviolet germicidal irradiation for water purification.
For color version of this figure, the reader is referred to the online version of
this book.
UVGI is employed to sterilize drinking and wastewater, as the holding facilities are
enclosed and can be circulated to ensure a higher exposure to the UV. UV sterilizers
are commonly used in aquarium circulation systems and hot tub circulation systems.
In recent years, UVGI has found renewed application in air sanitization.
Mode of Action: UV photons of different energies have various effects on DNA. The
most important damage to DNA is the formation of pyrimidine dimers, which form between
two adjacent pyrimidine bases—cytosine (C) and/or thymine (T) as shown in Fig. 1.18
. This abnormal link distorts the shape of the DNA double helix and blocks its copying
by the DNA replication or RNA transcription machinery. A block in either of these
important processes would be very dangerous for a cell; as little as one dimer per
cell in fact can be lethal. Dimers are formed in DNA most efficiently by UV-C, less
efficiently by UV-B, and very little by UV-A action. The chemical structural changes
are shown in Fig. 1.19
. UV-A light creates mostly free radicals that lead to indirect DNA damage.
7
Figure 1.18
Ultraviolet cross-linking of adjacent thymine dimers in a DNA/RNA strand.
7
Figure 1.19
Ultraviolet cross-linking structural details of adjacent thymine–cytosine in a DNA/RNA
strand on the left, with thymine–thymine dimerization or cross-linking on the right.
1.2.8
Microwave
Microwave sterilization
8
is primarily a thermal process. The heat is generated as microwaves interact with
polar water molecules and charged ions. The water molecules align rapidly in alternating
the electromagnetic field induced by the microwaves; the friction resulting from the
oscillating molecules generates the heat within food. The temperature achieved is
limited to about that of boiling water, unless the food is being held under pressure.
Because the heat is produced directly in the food, the thermal processing time is
sharply reduced compared to processes applying external heat. The food is not necessarily
cooked because the times are short. For commercial food applications, this is a developing
technology. The microwave sterilization technology developed at Washington State University
uses the combination of 915 MHz microwave and conventional heating to improve heating
uniformity. Commercial systems performing microwave pasteurization and/or sterilization
of foods are currently available in Europe
9
(e.g. TOP's Foods); however, the use of microwaves in the United States to produce
prepackaged shelf-stable foods is pending upon FDA acceptance.
Consumers have been able to use their microwaves to sterilize baby bottles in time
as short as 90 s. One such unit is shown in Fig. 1.20
. Steam from boiling water does the sterilizing.
Figure 1.20
Munchkin® Steam Guard Microwave Sterilizer. For color version of this figure, the
reader is referred to the online version of this book.
Mode of Action: The mechanism of germicidal action is essentially heating. Other possible
nonthermal mechanisms have been proposed. One of these is electroporation, cell membrane
damage and rupture, and direct electromagnetic energy coupling leading to cell lysis
(the destruction of cells by disruption of the bounding membrane, allowing the cell
contents to escape). However, scientific support for these mechanisms is not entirely
convincing.
1.2.9
Infrared
IR heating can be used to inactivate bacteria, spores, yeast, and mold in both liquid
and solid foods. Efficacy of microbial inactivation by IR heating depends on several
parameters including:
•
IR power level
•
Temperature of food sample
•
Peak wavelength of IR radiation
•
Bandwidth of IR heating source
•
Sample depth
•
Types of microorganisms
•
Moisture content
•
Types of food materials.
Inactivation mechanism by IR heating may include DNA damage in addition to thermal
effect. Thermal inactivation can damage DNA, RNA, ribosome, cell envelope, and proteins
in microbial cells.
1.2.10
How Does Irradiation Destroy Bacteria?
Radiation breaks the DNA or damages other critical molecules in bacteria, either killing
them or preventing them from reproducing. Irradiation is a process that employs ionizing
energy. Radiation energy ionizes a very small number of the molecules within the food
product and any bacteria within the food product. When a molecule is ionized, it effectively
breaks. If a DNA molecule of a bacterium is ionized, it is damaged or destroyed, preventing
the bacteria from being able to reproduce.
If a water molecule within a bacterium cell is ionized, it can form peroxide and act
as a disinfectant within the bacterium itself. This is covered later in this chapter.
These molecular level changes destroy the bacteria by preventing their reproduction.
Only a very small fraction of the molecules within a living organism, such as a bacterium,
need to be damaged to have this effect. Because only a small fraction of food product
molecules are ionized, minimal adverse effects, such as changes in taste, occur.
1.2.11
Doses of Radiation Required
Radiation dose is the quantity of radiation energy absorbed by the food as it passes
through the radiation field during processing. International health and safety authorities
have endorsed the safety of irradiation for all foods up to a dose level of 10,000 Gy
(10 kGy). The bactericidal efficacy of a given dose of irradiation depends on the
following
10
:
•
The kind and species of the organism (a few are listed in Table 1.6
).
Table 1.6
Approximate Killing Doses of Ionizing Radiations in Kilograys (kGy)
10
Organism
Approximate Lethal Dose (kGy)
Insects
0.22–0.93
Viruses
10–40
Yeasts (fermentative)
4–9
Yeasts (film)
3.7–18
Molds (with spores)
1.3–11
Bacteria
Mycobacterium tuberculosis
1.4
Staphylococcus aureus
1.4–7.0
Corynebacterium diphtheriae
4.2
Salmonella species
3.7–4.8
Escherichia coli
1.0–2.3
Pseudomonas aeruginosa
1.6–2.3
Pseudomonas fluorescens
1.2–2.3
Enterobacter aerogenes
1.4–1.8
Lactobacillus spp.
0.23–0.38
Streptococcus faecalis
1.7–8.8
Leuconostoc dextranicum
0.9
Sarcina lutea
3.7
Bacterial spores
Bacillus subtilis
12–18
Bacillus coagulans
10
Clostridium botulinum (A)
19–37
Clostridium botulinum (E)
15–18
Clostridium perfringens
3.1
Putrefactive anaerobe 3679
23.50
Bacillus stearothermophilus
10–17
•
The numbers of organisms (or spores) originally present. The more organisms there
are, the less effective a given dose will be.
•
The composition of the food. Some constituents may be protective. Compounds that combine
with the SH groups would be sensitizing.
•
The presence or absence of oxygen. The effect of free oxygen varies with the organism,
ranging from no effect to sensitization of the organism. Undesirable “side reactions”
are likely to be intensified in the presence of oxygen and to be less frequent in
a vacuum.
•
The physical state of the food during irradiation. Both moisture content and temperature
affect different organisms in different ways.
•
The condition of the organisms. Age, temperature of growth and sporulation, and state
(vegetative or spore) may affect the sensitivity of the organisms.
1.2.12
Dosimetry
The success of radiation processing of food depends to a large extent on the ability
of the irradiation processor:
•
To measure the absorbed dose delivered to the food product (through reliable dosimetry)
•
To determine the dose distribution patterns in the product package (through process
qualification procedures)
•
To control the routine radiation process (through process control procedures).
This subject is well documented in the literature and will not be discussed further
here.
11
1.3
Medical Sterilization
Until the latter half of the nineteenth century patients undergoing even the most
routine operations were at very high risk of infection because surgery was not performed
under sterile conditions. The operating room, the surgeon's hands, and the surgical
instruments were laden with microbes, which caused high levels of infection and mortality.
At the time bad or contaminated air was believed to be the cause of infection. It
was not until French scientist Louis Pasteur demonstrated that invisible microbes
caused disease, that elimination of them in medical facilities was recognized by numerous
doctors.
Ignaz Philipp Semmelweis was a Hungarian physician now known as an early pioneer of
antiseptic procedures. Described as the “savior of mothers,” Semmelweis discovered
that the incidence of puerperal fever could be drastically cut by the use of hand
disinfection in obstetrical clinics. Puerperal fever, also known as childbed fever,
is a bacterial infection contracted by women during childbirth or miscarriage. It
can develop into puerperal sepsis, which is a serious form of septicemia. It was often
fatal in mid-nineteenth century hospitals with mortality at 10–35%. Semmelweis proposed
washing with chlorinated lime solutions in 1847. He published a book of his findings:
Etiology, Concept, and Prophylaxis of Childbed Fever.
Heinrich Hermann Robert Koch was a German physician who became famous for isolating
Bacillus anthracis (1877), the Tuberculosis bacillus (1882), and Vibrio cholerae (1883)
and for his development of Koch's postulates, which were four criteria to identify
the causative agent of a particular disease. He is considered one of the founders
of microbiology, inspiring such major figures as Paul Ehrlich and Gerhard Domagk.
Joseph Lister is often considered to be the father of modern surgery, who promoted
the idea of sterile surgery while working at the Glasgow Royal Infirmary. Until Lister's
studies of surgery, most people believed that chemical damage from exposure to bad
air, “miasma,” was responsible for infections in wounds. Hospital wards were occasionally
aired out at midday as a precaution against the spread of infection via miasma. Hands
or a patient's wounds were not washed. While he was a professor of surgery at the
University of Glasgow, Lister read a paper published by the French chemist Louis Pasteur,
which showed that rotting and fermentation could occur under anaerobic conditions
if microorganisms were present. Pasteur suggested three methods to eliminate the microorganisms
responsible for gangrene: filtration, exposure to heat, or exposure to chemical solutions.
Lister confirmed Pasteur's conclusions with his own experiments and decided to use
his findings to develop antiseptic techniques for wounds. Lister successfully introduced
carbolic acid (now known as phenol) to sterilize surgical instruments and to clean
wounds, which led to reducing postoperative infections and made surgery safer for
patients.
Therefore, Lister tested the results of spraying instruments, the surgical incisions,
and dressings with a solution of it. Lister found that carbolic acid solution swabbed
on wounds remarkably reduced the incidence of gangrene. In August 1865, Lister applied
a piece of lint dipped in carbolic acid solution onto the wound of an 11-year-old
boy at Glasgow Infirmary, who had sustained a compound fracture after a cart wheel
had passed over his leg. After 4 days, he renewed the pad and discovered that no infection
had developed, and after a total of 6 weeks, he was amazed to discover that the boy's
bones had fused back together, without the danger of formation or discharge of pus.
At that time, the mortality rate of a compound fracture was about 60%. He instructed
surgeons under his responsibility to wear clean gloves and wash their hands before
and after operations with 5% carbolic acid solutions. Instruments were also washed
in the same solution and assistants sprayed the solution into the air in the operating
theatre.
As the germ theory of disease became more widely accepted, it was realized that infection
could be better avoided by preventing bacteria from getting into wounds in the first
place. This led to the rise of sterile surgery. Some consider Lister “the father of
modern antisepsis.” In 1879, Listerine mouthwash was named after him for his work
in antisepsis. Also named in his honor is the bacterial genus Listeria, typified by
the food-borne pathogen Listeria monocytogenes.
Hospitals and other medical treatment facilities are now concerned about sterilization.
What is sterilization? According to the CDC (Centers for Disease Control and Prevention),
“Sterilization means the use of a physical or chemical procedure to destroy all microbial
life, including highly resistant bacterial endospores.” The processes used to kill
microbiological life can affect the materials that are exposed to these processes.
For example, when food is irradiated it is often packaged in plastic materials. How
does the irradiation process affect the properties of the packaging? When medical
devices and facilities are sterilized those processes may affect the plastics used
in providing medical care. The plastics may be implants, surgical tools and supplies,
or packaging for these items. This book is focused on characterizing and understanding
how materials change when they are irradiated or sterilized.
1.3.1
Sterilization, Disinfection, and Asepsis
Medical sterilization is a much broader field than food irradiation. Three terms are
often used when talking about this subject and they are: sterilization, disinfection,
and asepsis. Sterility and asepsis refer to different conditions. Sterility indicates
the elimination (death) of all viable life forms and their germinative elements such
as eggs, spores and endospores. Sterility is absolute; there is no such thing as an
object being “partially sterile.” Asepsis generally means that only certain types
of life forms have been removed, excluded or neutralized (rendered nonviable), while
the presence of other organisms may be tolerated or even promoted. For example, pasteurization
of food does not kill all microorganisms in the food; it does dramatically reduce
the number of microorganisms, with the intent that the pasteurized material is stored
in conditions that will maintain that low number, such as refrigeration. Disinfection
refers to a process whereby many or all pathogenic microorganisms are neutralized
or removed. Unlike sterilization, disinfection can be achieved at varying levels as
defined by the CDC:
•
High-level disinfectants are chemical sterilants that may be used for a shorter exposure
period than would be required for sterilization to kill all microorganisms with the
exception of high numbers of bacterial spores.
•
Intermediate-level disinfectants will kill mycobacteria (causes of tuberculosis and
leprosy), vegetative bacteria, most viruses (such as poliovirus), and most fungi but
do not kill all bacterial spores.
•
Low-level disinfectants kill most vegetative bacteria, some fungi, and some viruses
such as Staphylococcus species, Pseudomonas species, Salmonella species, HIV virus,
herpes simplex virus, hepatitis B, hepatitis C, and many common cold viruses.
There are hundreds of items that are routinely sterilized or disinfected. In terms
of health care/medical items they are often classified by Spaulding's Classification:
critical, semi-critical, and noncritical, as shown in Table 1.7
.
Table 1.7
Spaulding's Classification
Classification
Definition
Level of Processing Required
Critical equipment/device
Equipment/device that enters sterile tissues, including the vascular system
Cleaning followed by sterilization
Semi-critical equipment/device
Equipment/device that comes in contact with nonintact skin or mucous membranes but
do not penetrate them
Cleaning followed by high-level disinfection as minimum; sterilization if preferred
Noncritical equipment/device
Equipment/device that touches only intact skin and not mucous membranes or does not
directly touch the client/patient/resident
Cleaning followed by low-level disinfection. In some cases, cleaning alone is acceptable
Disinfection and sterilization are both decontamination processes. While disinfection
is the process of eliminating or reducing harmful microorganisms from inanimate objects
and surfaces, sterilization is the process of killing all microorganisms. Sterilization
also destroys the spores of various organisms present on surfaces, in liquids, in
medication, or in compounds such as biological culture media. Such “extreme” forms
of decontamination are needed during critical times like surgery, or in environments
like industrial, laboratory or hospital. It is more practical to use disinfection
in everyday life.
Disinfection is usually carried out using chemicals, often solutions, but also vapors
and gases. When chemicals are used to destroy all forms of microbiologic life, they
can be called chemical sterilants. These same germicides used for shorter exposure
periods also can be part of the disinfection process (i.e. high-level disinfection).
Sterilization falls into the following three basic categories of which there are several
options in each category:
1.
High temperature/pressure sterilization (autoclave)
2.
Chemical sterilization
3.
Radiation sterilization.
Each of these and several less common methods are discussed in later sections.
1.3.2
Thermal Methods of Sterilization
Heat is one of the oldest and cheapest ways to sterilize food, tools, equipment, etc.
There are several basic ways to utilize heat and these are described in the following
sections.
1.3.2.1
Steam Sterilization/Autoclave
The steam autoclave is the oldest, safest, most widely used and most cost-effective
method of sterilization in the medical equipment industry. Steam is generated in a
pressure chamber so that it reaches a temperature 121–148 °C (250–300 °F) at 15 p.s.i.
The time items are kept exposed is dependent on the temperature and size of load and
usually ranges from 10 to 60 min. There are two main types of steam autoclaves, gravity
displacement and pre-vacuum cycle.
Gravity displacement autoclaves remove air from the chamber by gravity displacement
as steam-entering chamber near the top exerts pressure on air forcing it out of the
bottom as shown in Fig. 1.21
. A photo of an industrial-size steam autoclave is shown in Fig. 1.22
.
Figure 1.21
Diagram of a gravity displacement steam autoclave (courtesy of Pearson Education).
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.22
Picture of a large industrial size autoclave. For color version of this figure, the
reader is referred to the online version of this book.
The high-speed pre-vacuum sterilizers are similar to the gravity displacement sterilizers
except they are fitted with a vacuum pump (or ejector) to ensure air removal from
the sterilizing chamber and load before the steam is admitted. The advantage of using
a vacuum pump is that there is nearly instantaneous steam penetration even into porous
loads.
Table 1.8
summarizes the processing parameters for each cycle of the two types of steam autoclaves:
Table 1.8
Operating Parameters for Steam Autoclaves
Configuration
Temperature
Time
Gravity displacement
121–123 °C (250–254 °F)
15–30 min
132–135 °C (270–272 °F)
10–25 min
Pre-vacuum
132–135 °C (270–272 °F)
3–4 min
Like all sterilization processes, steam sterilization may have some damaging effects
on some materials. At constant temperatures, sterilization times vary depending on
the type of item (e.g. metal versus rubber, plastic, items with lumens), whether the
item is wrapped or unwrapped, and the sterilizer type.
Mode of Action: Moist heat destroys microorganisms by the irreversible coagulation
and denaturation of enzymes and structural proteins. In support of this fact, it has
been found that the presence of moisture significantly affects the coagulation temperature
of proteins and the temperature at which microorganisms are destroyed.
Uses: Steam sterilization is used whenever possible on all critical and semi-critical
items that are heat and moisture resistant (e.g. steam sterilizable respiratory therapy
and anesthesia equipment), even when not essential to prevent pathogen transmission.
Steam sterilizers also are used in health care facilities to decontaminate microbiological
waste and sharps containers but additional exposure time is required in the gravity
displacement sterilizer for these items. Steam sterilizers are common in dental facilities.
Advantages:
•
Nontoxic to patient, staff, environment
•
Cycle easy to control and monitor
•
Rapidly microbicidal
•
Least affected by organic/inorganic soils among sterilization processes listed
•
Rapid cycle time
•
Penetrates medical packing, device lumens.
Disadvantages:
•
Deleterious for heat-sensitive instruments
•
Microsurgical instruments damaged by repeated exposure can corrode surgical alloys
and cutting edges
•
Development of pitting and dulling of the cutting edges after multiple steam sterilization
cycles
•
May leave instruments wet, causing them to rust
•
Potential for burns.
1.3.2.2
Flash Sterilization
Flash sterilization is often called immediate-use steam sterilization.
12
“Immediate use” is broadly defined as the shortest possible time between a sterilized
item's removal from the sterilizer and its aseptic transfer to the sterile field.
It is used for sterilizing cleaned patient-care items that cannot be packaged, sterilized,
and stored before use. It also is used when there is insufficient time to sterilize
an item by the preferred package method. “Flash” steam sterilization was originally
defined by Underwood and Perkins as sterilization of an unwrapped object at 132°C
for 3 min at 27–28 lbs of pressure in a gravity displacement sterilizer.
Often flash sterilization equipment is located very close to operating rooms to handle
urgent needs. However, care must be taken to avoid burns to both the medical staff
and the patient. Burns may be prevented by either air cooling the instruments or immersion
in a sterile liquid such as saline.
Uses: Flash sterilization should not be used for reasons of convenience. It is not
recommended for implantable devices except where unavoidable.
1.3.2.3
Dry Heat Sterilization
Dry heat is effective at inactivating microorganisms, but in general, the temperatures
required are higher than is required for steam sterilization to achieve an equivalent
level of germicidal action. This method should be used only for materials that might
be damaged by moist heat or that are impenetrable to moist heat (e.g. powders, petroleum
products, sharp instruments).
There are two types of dry heat sterilizers: the static-air type and the forced-air
type. The differences are like two types of ovens common in everyday home kitchens.
The static type is like a conventional oven and the forced-air type is like a convection
oven. The static-air type has heating coils in the bottom of the unit cause the hot
air to rise inside the chamber via convection. This type of dry heat sterilizer is
much slower in heating, requires longer time to reach sterilizing temperature, and
is less uniform in temperature control throughout the chamber than is the forced-air
type. The forced-air or mechanical convection sterilizer is equipped with a motor-driven
blower that circulates heated air throughout the chamber at a high velocity, permitting
a more rapid transfer of energy from the air to the instruments.
Common time–temperature relationships for sterilization with hot air sterilizers are:
•
170 °C (340 °F) for 60 min
•
160 °C (320 °F) for 120 min
•
150 °C (300 °F) for 150 min.
The advantages for dry heat include:
•
It is nontoxic
•
Does not harm the environment
•
Easy to install
•
Relatively low operating costs
•
It penetrates materials
•
It is noncorrosive for metal.
The main disadvantage is time. Dry heat penetration and microbial killing is time
consuming by this method. In addition, the high temperatures are not suitable for
most materials.
Mode of Action: The primary lethal process is considered to be oxidation of cell constituents.
1.3.2.4
Glass Bead Sterilizer
Glass bead “sterilization” uses small glass beads (1.2–1.5 mm diameter) and high temperature
(217–232 °C) for brief exposure times (e.g. 45 s) to inactivate microorganisms. They
are typically used on metal surgical instruments as shown in Fig. 1.23
. These devices have been used for several years in the dental profession. FDA believes
there is a risk of infection with this device because of potential failure to sterilize
dental instruments and their use should be discontinued until the device has received
FDA clearance.
Figure 1.23
Picture of a glass bead sterilizer (photo courtesy of Cellpoint Scientific Inc.).
For color version of this figure, the reader is referred to the online version of
this book.
1.3.2.5
Microwave
Microwaves are used in medicine for disinfection of soft contact lenses, dental instruments,
dentures, milk, and urinary catheters for intermittent self-catheterization. However,
microwaves must only be used with products that are compatible (e.g. do not melt).
Microwaves are radio frequency waves, which are usually used at a frequency of 2450 MHz.
The microwaves produce friction of water molecules in an alternating electrical field.
The intermolecular friction derived from the vibrations generates heat and some authors
believe that the effect of microwaves depends on the heat produced while others postulate
a nonthermal lethal effect. The initial reports showed microwaves to be an effective
microbicide. The microwaves produced by a “home-type” microwave oven (2.45 GHz) completely
inactivate bacterial cultures, mycobacteria, viruses, and Geobacillus stearothermophilus
spores within 60 s to 5 min depending on the organism. Another study confirmed these
results but also found that higher power microwaves in the presence of water may be
needed for sterilization. Microwaves used for sterilization of medical devices have
not been FDA cleared.
1.3.2.6
IR Radiation
An IR radiation prototype sterilizer was investigated and found to destroy Bacillus
atrophaeus spores. IR heating can be effectively used for enzyme inactivation. IR
heating can be used to inactivate bacteria, spores, yeast, and mold in both liquid
and solid foods. Efficacy of microbial inactivation by IR heating depends on the following
parameters: IR power level, temperature of food sample, peak wavelength, bandwidth
of IR heating source, sample depth, types of microorganisms, moisture content, and
types of food materials.
13
Some of the possible advantages of IR technology include short cycle time, low energy
consumption, no cycle residuals, and no toxicological or environmental effects. This
may provide an alternative technology for sterilization of selected heat-resistant
instruments but there are no FDA-cleared systems for use in health care facilities.
1.3.3
Chemical Disinfectants – Liquids
There are many liquid chemical disinfectants available.14., 15. Some of these may
be used in vapor form and that approach is discussed in the next section. All disinfectants
act by harming microorganisms in some manner. The different disinfectants have different
mechanisms of action. These mechanisms of harm include:
•
Protein denaturation
•
Membrane disruption
•
Nucleic acid damage
•
Inhibition of metabolism.
Protein denaturation
It is commonly defined as any noncovalent change in the structure of a protein. This
change may alter the secondary, tertiary, or quaternary structure of the molecules.
For those proteins that are enzymes, denaturation can be defined as the loss of enough
structure to render the enzyme inactive, see Fig. 1.24
.
Figure 1.24
Denaturation of a protein can change its shape, which can destroy its function.
Membrane disruption
The bacterial cytoplasmic membrane is composed of a phospholipid bilayer. It has all
the general functions of a cell membrane such as acting as a permeability barrier
for most molecules and serving as the location for the transport of molecules into
the cell. Membrane disruption by chemical disinfectants is the breakdown of that membrane,
which means loss of the permeability barrier and death to the microorganism.
Nucleic acid damage
DNA damage affects the primary structure of the double helix; that is, the bases themselves
are chemically modified. These modifications can in turn disrupt the molecules' regular
helical structure by introducing nonnative chemical bonds or bulky adducts that do
not fit in the standard double helix:
•
Oxidation of bases and generation of DNA strand interruptions from reactive oxygen
species
•
Alkylation of bases (usually methylation)
•
Hydrolysis of bases, such as deamination, depurination, and depyrimidination.
Inhibition of metabolism
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity.
Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance.
Many liquid chemical sterilants are discussed in the following sections. Generally
for each sterilant, a chemical description or structure is provided. The mode of action
is noted and described. Typical uses of the sterilants are listed as are the advantages
and disadvantages to it use are summarized.
1.3.3.1
Alcohol
In the health care setting, “alcohol” refers to two water-soluble chemical compounds—ethyl
alcohol and isopropyl alcohol. FDA has not cleared any liquid chemical sterilant or
high-level disinfectant with alcohol as the main active ingredient. These alcohols
are rapidly bactericidal against vegetative forms of bacteria, fungicidal (an agent
that destroys fungi), and virucidal (capable of neutralizing or destroying a virus).
Alcohols do not destroy bacterial spores. Their cidal activity drops sharply when
diluted below 50% concentration, and the optimum bactericidal concentration is 60–90%
solution in water (volume/volume).
Mode of Action: The most feasible explanation for the antimicrobial action of alcohol
is denaturation of proteins. Protein shape is critical to performance of the protein.
That shape is maintained by intermolecular forces such as hydrogen bonding (see Section
2.8.1). An example is shown in Fig. 1.25
which shows the hydrogen bonding between two amino acids.
Figure 1.25
Denaturation of hydrogen bonding between two amino acids (aspartic acid, tyrosine)
in a protein by ethanol.
16
Microbiocidal Activity: Methyl alcohol (methanol) has the weakest bactericidal action
of the alcohols and thus seldom is used in health care. The bactericidal activity
of various concentrations of ethyl alcohol (ethanol) and isopropanol ranges from 10 s
to hours depending on the microorganism.
Uses: A 70% alcohol solution is used as a disinfectant on the skin. This concentration
of alcohol is able to penetrate the bacterial cell wall and denature the proteins
and enzymes inside of the cell. A 95% alcohol solution merely coagulates the protein
on the outside of the cell wall and prevents any alcohol from entering the cell. Alcohols
are not recommended for sterilizing medical and surgical materials principally because
they lack sporicidal action and they cannot penetrate protein-rich materials. Alcohols
have been used effectively to disinfect oral and rectal thermometers, hospital pagers,
scissors, and stethoscopes. Alcohol towelettes have been used to disinfect small surfaces
such as rubber stoppers of multiple-dose medication vials or vaccine bottles. Furthermore,
alcohol occasionally is used to disinfect external surfaces of equipment (e.g. stethoscopes,
ventilators, and manual ventilation bags), Cardiopulmonary resuscitation (CPR) manikins,
ultrasound instruments or medication preparation areas. Alcohols may damage coatings,
plastics, and elastomers.
1.3.3.2
Chlorine and Chlorine Compounds
Chlorine's disinfecting effects are well known as it has been used to disinfect drinking
water for many years. Many chlorine compounds release chlorine and are used in the
health care setting. The advantage of these compounds over the hypochlorites is that
they retain chlorine longer and so exert a more prolonged bactericidal effect. Some
of these are discussed in the next sections.
1.3.3.2.1
Hypochlorites
Hypochlorites, the most widely used of the chlorine disinfectants, are available as
liquid (household bleach, sodium hypochlorite) or solid (common pool chlorine, calcium
hypochlorite). The hypochlorite ion, also known as chlorate (I) anion, is ClO−. Household
bleach is an aqueous solution of 5.25%–6.15% sodium hypochlorite.
Hypochlorites have a broad-spectrum of antimicrobial activity. They do not leave toxic
residues, are unaffected by water hardness, are inexpensive and fast acting. They
remove dried or fixed organisms and biofilms from surfaces and have a low incidence
of serious toxicity.
One danger is the release of toxic chlorine gas when mixed with ammonia or acid (e.g.
household cleaning agents). The microbicidal activity of chlorine is attributed largely
to undissociated hypochlorous acid (HOCl). The dissociation of HOCl to the less microbicidal
form (hypochlorite ion OCl−) depends on pH. The disinfecting efficacy of chlorine
decreases with an increase in pH that parallels the conversion of undissociated HOCl
to OCl−.
1.3.3.2.2
Chlorine dioxide
Chlorine dioxide (CD, ClO2) is greenish yellow and is an oxidizing agent with a chlorine-like
odor. ClO2 is effective against bacteria, yeasts, molds, and viruses. The rapid sterilizing
activity of ClO2 is present at ambient temperature and at relatively low gas concentration,
1–30 mg/l.
Traditionally, chlorine dioxide for disinfection applications is made where it is
to be used by one of three methods using sodium chlorite or the sodium chlorite–hypochlorite
method:
2
NaClO
2
+
2
HCl
+
NaOCl
→
2
ClO
2
+
3
NaCl
+
H
2
O
or the sodium chlorite–hydrochloric acid method:
5
NaClO
2
+
4
HCl
→
5
NaCl
+
4
ClO
2
+
2
H
2
O
Mode of Action: Chlorine dioxide acts as an oxidizing agent and reacts with several
cellular constituents, including the cell membrane of microbes. The potency of chlorine
dioxide is attributable to the simultaneous, oxidative attack on many proteins thereby
preventing the cells from mutating to a resistant form.
Uses: Drinking water, poultry process water, swimming pools, and mouthwash preparations.
It is used to sanitize fruit and vegetables as well as equipment for food and beverage
processing. It is used in the life sciences industry to decontaminate animal research
facilities. It is also employed in the health care industries to decontaminate rooms,
pass-throughs, isolators, and also as a sterilant for product and component sterilization.
1.3.3.2.3
Sodium dichloroisocyanurate
Sodium dichloroisocyanurate is used as a disinfectant in water. Its structure is shown
in Fig. 1.26
. It is considered to be a halogen donor, a chemical that releases active chlorine.
After release, the halogen reaction is similar to that of chlorine or bromine from
other sources. Chlorine donor chemicals do not release the active chlorine all at
once, but make it slowly available.
Figure 1.26
Chemical structure of sodium dichloroisocyanurate.
1.3.3.2.4
Chloramine-T
Chloramine-T, the structure of which is shown in Fig. 1.27
, is available in tablet or powder form and has to be dissolved before use. It is
sprayed on a surface and allowed to stand for at least 15 min before being wiped off
or allowed to dry.
Figure 1.27
Chemical structure of chloramine-T (N-chloro 4-methylbenzenesulfonamide, sodium salt).
Mode of Action: The molecular structure of chloramine-T is similar to para-aminobenzoic
acid which is an intermediate in bacterial metabolism. Chloramine-T disrupts bacterial
metabolism inhibiting growth. The hypochlorite moiety can destroy the DNA structure
via oxidation and thereby prevents microbes from reproducing.
1.3.3.2.5
Superoxidized water
“Superoxidized water” has been examined as a disinfectant. The concept of electrolyzing
saline to create a disinfectant or antiseptics is appealing because the basic materials
of saline and electricity are inexpensive and the end product (i.e. water) does not
damage the environment.
17
The main products of the electrolysis are hypochlorous acid (e.g. at a concentration
of about 144 mg/l) and chlorine. As with any germicide, the antimicrobial activity
of superoxidized water is strongly affected by the concentration of the active ingredient
(available free chlorine). One manufacturer, PuriCore, generates the disinfectant
at the point of use by passing a saline solution over coated titanium electrodes at
9 amps. The product generated has a pH of 5.0–6.5 and an oxidation–reduction potential
(redox) of >950 mV. Although superoxidized water is intended to be generated fresh
at the point of use, when tested under clean conditions, the disinfectant was effective
within 5 min when 48 h old. Unfortunately, the equipment required to produce the product
can be expensive because parameters such as pH, current, and redox potential must
be closely monitored. The solution is nontoxic to biologic tissues.
In October 2002, the FDA cleared superoxidized water as a high-level disinfectant
(FDA, personal communication, September 18, 2002).
Mode of Action: The exact mechanism by which free chlorine destroys microorganisms
has not been elucidated. Inactivation by chlorine can result from a number of factors:
oxidation of sulfhydryl enzymes and amino acids, ring chlorination of amino acids,
loss of intracellular contents, decreased uptake of nutrients, inhibition of protein
synthesis, decreased oxygen uptake, oxidation of respiratory components, decreased
adenosine triphosphate production, breaks in DNA, and depressed DNA synthesis. The
actual microbicidal mechanism of chlorine might involve a combination of these factors
or the effect of chlorine on critical sites.
Uses: Hypochlorites are widely used in health care facilities in a variety of settings.
1.3.3.3
Liquid Formaldehyde
Formaldehyde is used as a disinfectant and sterilant in both its liquid and gaseous
states. The gaseous method is discussed in a later section of this chapter. Formaldehyde
is sold and used principally as a water-based solution called formalin, which is 37%
formaldehyde by weight. The aqueous solution is a bactericide, tuberculocide, fungicide,
virucide, and sporicide.
Mode of Action: Formaldehyde is an extremely reactive chemical that interacts with
proteins, DNA, and RNA.
Formaldehyde inactivates microorganisms by alkylating the amino (–NH2) and sulfhydral
(–S–H) groups of proteins and ring nitrogen atoms of purine bases. This is shown in
Fig. 1.28
. The interaction with protein results from a combination with the primary amide as
well as with the amino groups. Formaldehyde acts as an alkylating agent by reaction
with carboxyl (–C=O), sulfhydryl (-SH), and hydroxyl (-OH) groups. Formaldehyde also
reacts extensively with nucleic acid. Two of the four bases in nucleic acids, adenine
2
and guanine,
3
are purines. In DNA, these bases form hydrogen bonds with their complementary pyrimidines,
thymine and cytosine, respectively. This is called complementary base pairing. In
RNA, the complement of adenine is uracil instead of thymine.
Figure 1.28
Alkylation by formaldehyde can lead to structural changes in proteins.
Uses: Although formaldehyde–alcohol is a chemical sterilant and formaldehyde is a
high-level disinfectant, the health care uses of formaldehyde are limited by its irritating
fumes and its pungent odor even at very low levels (<1 ppm). It is also a suspected
human carcinogen linked to leukemia, nasopharyngeal, and lung cancers. Formaldehyde
is used in the health care setting to prepare viral vaccines (e.g. poliovirus and
influenza), as an embalming agent, and to preserve anatomic specimens, and historically
has been used to sterilize surgical instruments, especially when mixed with ethanol.
1.3.3.4
Glutaraldehyde
Glutaraldehyde is a saturated dialdehyde that has gained wide acceptance as a high-level
disinfectant and chemical sterilant. Its chemical structure is shown in Fig. 1.29
. Aqueous solutions of glutaraldehyde are acidic and generally in this state are not
sporicidal. Only when the solution is “activated” (made alkaline) by use of alkalinating
agents to pH 7.5–8.5 does the solution become sporicidal. Once activated, these solutions
have a shelf life of minimally 14 days because of the polymerization of the glutaraldehyde
molecules at alkaline pH levels. This polymerization blocks the active sites (aldehyde
groups) of the glutaraldehyde molecules that are responsible for its biocidal activity.
Figure 1.29
Chemical structure of glutaraldehyde.
18
Mode of Action: Like formaldehyde, the biocidal activity of glutaraldehyde results
from its alkylation of sulfhydryl, hydroxyl, carboxyl, and amino groups of microorganisms,
which alters RNA, DNA, and protein synthesis.
Uses: Glutaraldehyde is used most commonly as a high-level disinfectant for medical
equipment such as endoscopes, spirometry tubing, dialyzers, transducers, anesthesia
and respiratory therapy equipment, hemodialysis proportioning and dialysate delivery
systems, and reuse of laparoscopic disposable plastic trocars. Glutaraldehyde is noncorrosive
to metal and does not damage lenses in instruments, rubber, or plastics.
Advantages:
•
Numerous use studies published
•
Relatively inexpensive
•
Excellent materials compatibility.
Disadvantages:
•
Respiratory irritation from glutaraldehyde vapor
•
Pungent and irritating odor
•
Relatively slow mycobactericidal activity
•
Coagulates blood and fixes tissue to surfaces
•
Allergic contact dermatitis
•
Glutaraldehyde vapor monitoring recommended.
1.3.3.5
Hydrogen Peroxide
Good germicidal activity has been ascribed to hydrogen peroxide; it has bactericidal,
virucidal, sporicidal, and fungicidal properties. The FDA website lists cleared liquid
chemical sterilants and high-level disinfectants containing hydrogen peroxide and
their cleared contact conditions.
Mode of Action: Hydrogen peroxide works by producing destructive hydroxyl-free radicals
that can attack membrane lipids, DNA, and other essential cell components. Catalase,
produced by aerobic organisms and facultative anaerobes that possess cytochrome systems,
can protect cells from metabolically produced hydrogen peroxide by degrading hydrogen
peroxide to water and oxygen. This defense is overwhelmed by the concentrations used
for disinfection. It acts on the microorganisms through its release of nascent oxygen.
Hydrogen peroxide produces hydroxyl-free radical that damages proteins and DNA.
Advantages:
•
No activation required
•
May enhance removal of organic matter and organisms
•
No disposal issues
•
No odor or irritation issues
•
Does not coagulate blood or fix tissues to surfaces
•
Inactivates Cryptosporidium
•
Use studies published.
Disadvantages:
•
Material compatibility concerns (brass, zinc, copper, and nickel/silver plating) both
cosmetic and functional
•
Serious eye damage with contact.
1.3.3.6
Iodophors
Iodine solutions or tinctures long have been used by health professionals primarily
as antiseptics on skin or tissue. Iodophors, on the other hand, have been used both
as antiseptics and disinfectants. An iodophor is a preparation containing iodine complexed
with a solubilizing agent, such as a surfactant or povidone. The structure of povidone–iodine
(PVP-I) is shown in Fig. 1.30
. A well-known PVP-I product is Betadine®. The result is a water-soluble material
that releases free iodine when in solution. Iodine is the active ingredient.
Figure 1.30
Structure of povidone–iodine.
Mode of Action: Iodine can penetrate the cell wall of microorganisms quickly, and
the lethal effects are believed to result from disruption of protein and nucleic acid
structure and synthesis.
Free iodine, the active ingredient in PVP-I, is rapidly lethal to bacteria, fungi,
viruses, and protozoa. These microbial effects are the result of cell wall penetration,
oxidation, and substitution of microbial contents with free iodine. It has strong
oxidizing effects on the functional groups of amino acids and fatty acids, particularly
the -NH2 and -SH groups of amino acids and the double bonds of fatty acids. Iodine
reaction with these groups leads to rapid damage to bacterial and fungal cells.
Uses: Besides their use as an antiseptic, iodophors have been used for disinfecting
blood culture bottles and medical equipment, such as hydrotherapy tanks, thermometers,
and endoscopes. Iodophors formulated as antiseptics contain less free iodine than
do those formulated as disinfectants.
1.3.3.7
Ortho-Phthalaldehyde
Ortho-phthalaldehyde (OPA) is a high-level disinfectant that received FDA clearance
in 1999. It contains 0.55% 1,2-benzenedicarboxaldehyde (OPA), the structure of which
is shown in Fig. 1.31
. OPA solution is a clear, pale blue liquid with a pH of 7.5. One popular commercial
formulation is Cidex® OPA.
19
Figure 1.31
Structure of ortho-phthalaldehyde.
Mode of Action: Preliminary studies on the mode of action of OPA suggest that it interacts
with amino acids, proteins, and microorganisms. However, OPA is a less potent cross-linking
agent that glutaraldehyde. However, the lipophilic aromatic nature of OPA allows its
uptake through the outer layers of mycobacteria and Gram-negative bacteria. OPA appears
to kill spores by blocking the spore germination process.
Uses: It is used to sterilize a wide range of medical devices.
Advantages:
•
Fast-acting high-level disinfectant
•
No activation required
•
Odor not significant
•
Excellent materials compatibility claimed
•
Does not coagulate blood or fix tissues to surfaces claimed.
Disadvantages:
•
Stains skin, mucous membranes, clothing, and environmental surfaces
•
Repeated exposure may result in hypersensitivity in some patients with bladder cancer
•
More expensive than glutaraldehyde
•
Eye irritation with contact
•
Slow sporicidal activity.
1.3.3.8
Peracetic Acid
Peracetic, or peroxyacetic acid (PAA, see Fig. 1.32
), is characterized by rapid action against all microorganisms. The sterilant, 35%
peracetic acid, and an anticorrosive agent are supplied in a single-dose container.
Special advantages of peracetic acid are that it lacks harmful decomposition products
(i.e. acetic acid, water, oxygen, hydrogen peroxide), enhances removal of organic
material, and leaves no residue. It remains effective in the presence of organic matter
and is sporicidal even at low-temperatures. It is considered unstable, particularly
when diluted; for example, a 1% solution loses half its strength through hydrolysis
in 6 days, whereas 40% peracetic acid loses 1–2% of its active ingredients per month.
One of the common formulations is Envirotech's Perasan®.
Figure 1.32
Peracetic acid is at equilibrium with acetic acid and hydrogen peroxide in water solutions.
Mode of Action: Little is known about the mechanism of action of peracetic acid, but
it is believed to function similarly to other oxidizing agents—that is, it denatures
proteins, disrupts the cell wall permeability, and oxidizes sulfhydryl and sulfur
bonds in proteins, enzymes, and other metabolites.
Advantages:
•
Rapid sterilization cycle time (30–45 min)
•
Low-temperature (50–55 °C) liquid immersion sterilization
•
Environmental friendly by-products (acetic acid, O2, H2O)
•
Fully automated
•
Single-use system eliminates need for concentration testing
•
Standardized cycle
•
May enhance removal of organic material and endotoxin
•
No adverse health effects to operators under normal operating conditions
•
Compatible with many materials and instruments
•
Does not coagulate blood or fix tissues to surfaces
•
Sterilant flows through scope facilitating salt, protein, and microbe removal
•
Rapidly sporicidal
•
Provides procedure standardization (constant dilution, perfusion of channel, temperatures,
exposure)
•
PAA breaks down in food to safe and environmentally friendly residues (acetic acid
and hydrogen peroxide) and therefore can be used in nonrinse applications.
Disadvantages:
•
Potential material incompatibility (e.g. aluminum anodized coating becomes dull).
Peracetic acid can corrode copper, brass, bronze, plain steel, and galvanized iron
but these effects can be reduced by additives and pH modifications.
•
Used for immersible instruments only
•
Biological indicator may not be suitable for routine monitoring
•
One scope or a small number of instruments can be processed in a cycle
•
More expensive (endoscope repairs, operating costs, purchase costs) than high-level
disinfection
•
Serious eye and skin damage (concentrated solution) with contact
•
Point-of-use system, no sterile storage.
1.3.3.9
Peracetic Acid and Hydrogen Peroxide
As shown in Fig. 1.32, peracetic acid may be at equilibrium with hydrogen peroxide
and acetic acid. Formulations of the three ingredients are blended and used.
Uses: The combination of peracetic acid and hydrogen peroxide has been used for disinfecting
hemodialyzers.
Advantages:
•
No activation required
•
Odor or irritation not significant.
Disadvantages
•
Materials compatibility concerns (lead, brass, copper, zinc) both cosmetic and functional
•
Limited clinical experience
•
Potential for eye and skin damage.
1.3.3.10
Phenolics
Phenol is probably the oldest known disinfectant as it was first used by Lister, when
it was called carbolic acid. However, there are many phenol-based materials used as
disinfectants. Phenol derivatives originate when a functional group (e.g. alkyl, phenyl,
benzyl, halogen) replaces one of the hydrogen atoms on the aromatic ring.
•
o-Phenylphenol is often used instead of phenol since it is somewhat less corrosive.
The primary use of 2-phenylphenol is as an agricultural fungicide. It is generally
applied postharvest. It is a fungicide used for waxing citrus fruits.
•
Chloroxylenol (4-chloro-3,5-dimethylphenol) is the principal ingredient in Dettol,
a household disinfectant and antiseptic.
•
Hexachlorophene, also known as Nabac, is a phenolic that was once used as a germicidal
additive to some household products but was banned due to suspected harmful effects.
•
Thymol, derived from the herb thyme, is the active ingredient in some “broad-spectrum”
disinfectants that bear ecological claims. This antibacterial activity is caused by
inhibiting growth and lactate production and by decreasing cellular glucose uptake.
•
Amylmetacresol is found in Strepsils, a throat disinfectant.
The chemical structures of these materials are shown in Fig. 1.33
.
Figure 1.33
Structures of several phenol-based disinfectants.
The antimicrobial properties of these compounds and many other phenol derivatives
are much improved over those of the parent chemical. Phenolics are absorbed by porous
materials, and the residual disinfectant can irritate tissue.
Mode of Action: In high concentrations, phenol acts as a gross protoplasmic poison,
penetrating and disrupting the cell wall and precipitating the cell proteins. Low
concentrations of phenol and higher molecular weight phenol derivatives cause bacterial
death by inactivation of essential enzyme systems and leakage of essential metabolites
from the cell wall. Phenolics generally have poor effectiveness against endospores.
Uses: Many phenolic germicides are EPA registered as disinfectants for use on environmental
surfaces (e.g. bedside tables, bedrails, and laboratory surfaces) and noncritical
medical devices. Phenolics are not FDA cleared as high-level disinfectants for use
with semi-critical items but could be used to preclean or decontaminate critical and
semi-critical devices before terminal sterilization or high-level disinfection.
1.3.3.11
Quaternary Ammonium Compounds
Quaternary ammonium compounds (quats), such as benzalkonium chloride (see Fig. 1.34
for the chemical structure), are a large group of related compounds. Some concentrated
formulations have been shown to be effective low-level disinfectants. Typically, quats
do not exhibit efficacy against difficult-to-kill nonenveloped viruses such as norovirus,
rotavirus, or poliovirus. Newer synergous, low-alcohol formulations are highly effective
broad-spectrum disinfectants with quick contact times (3–5 min) against bacteria,
enveloped viruses, pathogenic fungi, and myco-bacteria.
Figure 1.34
Chemical structure of benzalkonium chloride compounds.
The quaternary ammonium compounds are widely used as disinfectants. Some of the chemical
names of quaternary ammonium compounds used in health care are alkyl dimethyl benzyl
ammonium chloride, alkyl didecyl dimethyl ammonium chloride, and dialkyl dimethyl
ammonium chloride. The newer quaternary ammonium compounds (i.e. fourth generation),
referred to as twin-chain or dialkyl quaternaries (e.g. didecyl dimethyl ammonium
bromide and dioctyl dimethyl ammonium bromide), purportedly remain active in hard
water and are tolerant of anionic residues.
Mode of Action: The bactericidal action of the quaternaries has been attributed to
the inactivation of energy-producing enzymes, denaturation of essential cell proteins,
and disruption of the cell membrane. Evidence exists that supports these and other
possibilities. The mechanism of bactericidal/microbicidal action is thought to be
due to disruption of intermolecular interactions. Quaternary ammonium compounds are
not effective against endospores.
Uses: The quaternaries commonly are used in ordinary environmental sanitation of noncritical
surfaces, such as floors, furniture, and walls. EPA-registered quaternary ammonium
compounds are appropriate to use for disinfecting medical equipment that contacts
intact skin (e.g. blood pressure cuffs).
1.3.3.12
Surfacine
Surfacine Development Company has introduced Surfacine, a persistent antimicrobial
agent that may be used on animate or inanimate surfaces. It incorporates a water-insoluble
antimicrobial-drug compound (silver iodide) incorporated in a surface-immobilized
coating (a modified polyhexamethylene biguanide). Microbial contact with the surface
results in transfer of the silver directly from the coating to the organism. Microorganisms
contacting the coating accumulate silver until the toxicity threshold is exceeded;
dead microorganisms eventually lyse and detach from the surface.
Advantages: It is persistent and has demonstrated vancomycin-resistant Enterococcus
species, methicillin-resistant Staphylococcus aureus, and Clostridium difficile.
20
1.3.3.13
Controlling Biofilm
Biofilm was mentioned in 1.1, 1.2 of this chapter. Studies have shown that routine
biocides such as chlorine, quats, peracetic acid, chlorine dioxide, and other oxidizers
are ineffective against bacteria that live in biofilm. While these agents may be able
to kill free-swimming pathogens, these dangerous bacteria will inevitably form biofilms
and become resistant to conventional treatment methods.
1.3.4
Gas and Vapor Sterilizing
There are many other sterilization techniques that use gas. Technically, steam could
be included as a gas or vapor. Some of the methods discussed in this chapter are used
to sterilize medical devices that are already packaged. These may be called terminally
sterilized medical devices. ISO 11607-1 details the fundamental attributes required
of materials and preformed systems intended for use in packaging systems for terminally
sterilized medical devices. ISO 11607-2 describes the validation requirements for
forming, sealing, and assembly processes. The development and validation of packaging
processes are crucial to ensure that sterile barrier system integrity is maintained
until opened by the users of sterile medical devices.
Goals of a terminally sterilized medical device packaging system include:
•
Allow sterilization through the packaging. For gaseous sterilants, this means the
gas must be able to permeation rapidly through the packaging.
•
Provide physical protection during handling, storage, and distribution
•
Maintain sterility to the point of use for a specific amount of time
•
Allow aseptic presentation
The ASTM tests for packaging include:
•
ASTM F1886 Standard Test Method for Determining Integrity of Seals for Medical Packaging
by Visual Inspection
•
ASTM F88 Standard Test Method for Seal Strength of Flexible Barrier Materials, which
is a peel Strength Test
•
ASTM F1140 Standard Test Methods for Internal Pressurization Failure Resistance of
Unrestrained Packages, which is a Burst Test
•
ASTM F1929 Standard Test Method for Detecting Seal Leaks in Porous Medical Packaging
by Dye Penetration
•
ASTM F1140 Standard Test Methods for Internal Pressurization Failure Resistance of
Unrestrained Packages
•
ASTM F2096 Standard Test Method for Detecting Gross Leaks in Medical Packaging by
Internal Pressurization (Bubble Test)
So in these cases, the effect of the sterilant on both the packaging and the device
must be con-sidered.
1.3.4.1
Ethylene Oxide Sterilization
Ethylene oxide (EtO) sterilization is mainly used to sterilize medical and pharmaceutical
products that cannot support conventional high temperature steam sterilization – such
as devices that incorporate electronic components, plastic packaging or plastic containers.
EtO gas infiltrates packages as well as products themselves to kill microorganisms
that are left during production or packaging processes. This gas, mixed with air at
a ratio of at least 3% EtO gas, forms an explosive mixture. The pure EtO gas boiling
point is 10.73 °C at atmospheric pressure. Most of the time, it is mixed with nitrogen
or carbon dioxide (CO2).
Most EtO sterilization lines involve three different stages.
1.
Preconditioning
2.
Sterilizer
3.
Degasser.
1.3.4.1.1
Preconditioning Stage
First, products need to go through a preconditioning phase to make microorganisms
grow. The batch load goes through a dwell time under a controlled environment of temperature
and humidity. This is often done in an environmentally controlled room. This part
of the process insures the sterilization process will be reproducible.
1.3.4.1.2
Sterilizer Stage
Then the load goes through a long and complex sterilization cycle. Requirements of
such a system are:
•
Accurate temperature control
•
Accurate pressure and vacuum control.
The sterilization phases are:
1.
Initial vacuum phase air must be removed or purged from the sterilization chamber
because mixtures of EtO and air can be explosive. For those items that can withstand
very low pressure, a vacuum is drawn on the chamber at a controlled rate. Being that
many of the items being sterilized are inside a package, a slow evacuation rate is
used when that packaging is not very permeable to the components in air. For those
devices, components and packaging that are not designed to withstand deep vacuums
and/or high pressures, a different approach to air remove is done. A shallow vacuum
with nitrogen gas purge is used. An initial shallow vacuum is drawn followed by a
nitrogen injection. The combination of the vacuum and nitrogen injection is called
a nitrogen wash. The nitrogen wash is repeated several times to assure an adequate
removal of air from the vessel.
2.
Humidification – When the initial evacuation phase of the process is performed, the
product can lose a significant amount of moisture. Moisture is required for the EtO
to sterilize properly. The lost moisture must be replaced prior to introducing the
ethylene oxide. This is accomplished by adding humidity in the form of steam injections.
The amount of steam required is calculated to yield a predetermined relative humidity.
After the addition of steam, the product is allowed to dwell or soak for the amount
of time required to replace the moisture lost from the evacuation phase.
3.
Liquid ethylene oxide is first heated into a gaseous phase and then injected into
the chamber. It is often “diluted” with a carrier gas such as Freon [hydrochlorofluorocarbon
(HCFC)]. Common dilutions are 8.6% EtO/91.4% HCFC, 10% EtO/90% HCFC, and 8.5% EtO/91.5%
CO2. The amount of gas or gas concentration is dependent on two primary factors that
are addressed during cycle design but it is usually 450–1200 mg/l. The most important
factor is to assure that the minimum gas concentration required to achieve sterility
within the product is attained. This minimum concentration must be balanced against
the second factor, which is the maximum amount of gas that can be injected before
difficulties arise due to high levels of poststerilization EtO residuals (EtO remaining
in the items being sterilized). Additionally, the chamber is kept at a temperature
of 37–63 °C and the relative humidity is 40–80%. Exposure time to EtO gas is typically
1–6 h.
1.3.4.1.3
Degassing Stage
4.
After the EtO exposure phase of the process, all gas must be removed from the chamber
until the levels of EtO fall below the flammable limit for the gas (3% or 30,000 ppm).
This is accomplished by performing a series of post-vacuums, each followed with a
nitrogen backfill (wash).
5.
After the process, removal of residual EtO is required. This is often done by aeration
at elevated temperature. Depending on the substrate materials can take 8–12 h or more.
Sometimes this is done in a special room but that may require 7 days if the temperature
is around 20 °C.
Mode of Action: The microbicidal activity of EtO is considered to be the result of
alkylation of protein, DNA, and RNA. Alkylation or the replacement of a hydrogen atom
with an alkyl group, within cells prevents normal cellular metabolism and replication.
Ethylene oxide acts more strongly against bacteria, especially Gram-positive bacteria,
than against yeast and fungi. The disinfectant effect of ethylene oxide is similar
to that of sterilization by heat, but because of limited penetration, it affects only
the surface.
Uses: EtO is used in health care facilities to sterilize critical items (and sometimes
semi-critical items) that are moisture or heat sensitive and cannot be sterilized
by steam sterilization.
Advantages:
For 100% EtO
•
Penetrates packaging materials, device lumens
•
Single-dose cartridge and negative-pressure chamber minimizes the potential for gas
leak and EtO exposure
•
Simple to operate and monitor
•
Compatible with most medical materials.
For EtO mixtures
•
Penetrates medical packaging and many plastics
•
Compatible with most medical materials
•
Cycle easy to control and monitor.
Disadvantages:
For 100% EtO
•
Requires aeration time to remove EtO residue
•
Sterilization chamber size from 4.0 to 7.9 ft
3
total volume (varies with model type)
•
EtO is toxic, a carcinogen, and flammable
•
EtO emission regulated by states but catalytic cell removes 99.9% of EtO and converts
it to CO2 and H2O
•
EtO cartridges should be stored in flammable liquid storage cabinet
•
Lengthy cycle/aeration time.
For EtO mixtures
•
Some states (e.g. CA, NY, MI) require EtO emission reduction of 90–99.9%
•
CFC (inert gas that eliminates explosion hazard) banned in 1995
•
Potential hazards to staff and patients
•
Lengthy cycle/aeration time
•
EtO is toxic, a carcinogen, and flammable.
1.3.4.2
Vaporized Hydrogen Peroxide
Hydrogen peroxide solutions have been used as chemical sterilants for many years.
However, the vaporized hydrogen peroxide (HPV or VHP®) approach was not developed
for the sterilization of medical equipment and spaces until the mid-1980s. One method
for delivering HPV uses a deep vacuum to pull liquid hydrogen peroxide (30–35% concentration)
from a disposable cartridge through a heated vaporizer and then, following vaporization,
into a sterilization chamber or space. A second approach to HPV delivery is the flow-through
approach in which the VHP is carried into the sterilization chamber or space by a
carrier gas such as air using either a slight negative pressure (vacuum) or slight
positive pressure.
Applications of this technology include vacuum systems for industrial sterilization
of medical devices and atmospheric systems for decontaminating for large and small
areas, such as laboratory workstations, isolation and pass-through rooms, and even
aircraft interiors. VHP offers several appealing features that include rapid cycle
time (e.g. 30–45 min), low-temperature, environmentally safe by-products (water, oxygen),
good material compatibility, and ease of operation, installation, and monitoring.
VHP has limitations including that cellulose cannot be processed, nylon becomes brittle,
and VHP penetration capabilities are less than those of EtO. VHP has not been cleared
by FDA for sterilization of medical devices in health care facilities.
Mode of Action: See section for a discussion on the mode of action of hydrogen peroxide.
1.3.4.3
Low-Temperature Hydrogen Peroxide Gas Plasma
New sterilization technology based on plasma was patented in 1987 and marketed in
the United States in 1993.
21
Gas plasmas have been referred to as the fourth state of matter (i.e. liquids, solids,
gases, and gas plasmas). Gas plasmas are generated in an enclosed chamber under deep
vacuum using RF or microwave energy to excite the gas molecules and produce charged
particles, many of which are in the form of free radicals. The five stages of the
process consist of:
1.
Vacuum
2.
Hydrogen peroxide (H2O2) injection
3.
Diffusion
4.
Plasma
5.
Vent.
The sterilization chamber is evacuated to 0.3 mmHg pressure. Hydrogen peroxide solution
is injected from a cassette and is vaporized in the sterilization chamber to a concentration
of 6 mg/l. The hydrogen peroxide vapor diffuses through the chamber for about 50 min
and exposes all surfaces and initiates the inactivation of microorganisms. At the
completion of the diffusion phase, the chamber pressure is reduced to 0.5 torr. An
electrical field created by an RF is applied to the chamber to create a gas plasma,
which lasts for 15 min. Microbicidal free radicals are generated in the plasma. The
excess gas is removed and in the final stage of the process the sterilization chamber
is returned to atmospheric pressure by introduction of high-efficiency filtered air.
The vapor purged from the chamber is vented to the atmosphere through a catalytic
filter to decompose all remaining traces of hydrogen peroxide into water and oxygen
vapor. The by-products of the cycle (e.g. water vapor, oxygen) are nontoxic and eliminate
the need for aeration. The process operates in the temperature range of 37–44 °C and
has a cycle time of 75 min. Multiple plasma cycles are sometimes used. Advanced Sterilization
Products' STERRAD® systems utilize this technology.
Mode of Action: Gas plasmas generate charged particles and radicals (hydroxyl and
hydroperoxyl free radicals). A free radical is an atom with an unpaired electron and
is a highly reactive species. The proposed mechanism of action of low-temperature
hydrogen peroxide gas plasma is the production of free radicals within a plasma field
that are capable of interacting with essential cell components such as enzymes and
nucleic acids. This disrupts the metabolism of microorganisms.
Uses: Materials and devices that cannot tolerate high temperatures and humidity, such
as some plastics, electrical devices, and corrosion-susceptible metal alloys, can
be sterilized by hydrogen peroxide gas plasma. This method has been compatible with
most (>95%) medical devices and materials tested.
Advantages:
•
Safe for the environment
•
Leaves no toxic residuals
•
Cycle time is 28–75 min (varies with model type) and no aeration necessary
•
Used for heat- and moisture-sensitive items since process temperature <50 °C
•
Simple to operate
•
Compatible with most medical devices
•
Only requires electrical outlet.
Disadvantages:
•
Cellulose (paper), linens, and liquids cannot be processed
•
Sterilization chamber size from 1.8 to 9.4 ft
3
total volume
•
Some endoscopes or medical devices with long or narrow lumens cannot be processed
at this time in the United States
•
Requires synthetic packaging (polypropylene wraps, polyolefin pouches) and special
container tray
•
Hydrogen peroxide may be toxic at levels greater than 1 ppm time-weighted average.
1.3.4.4
Formaldehyde Gaseous Sterilization
Paraformaldehyde, a solid polymer of formaldehyde, can be vaporized by heat for the
gaseous decontamination of laminar flow biologic safety cabinets when maintenance
work or filter changes require access to the sealed portion of the cabinet. This is
shown in Fig. 1.35
.
Figure 1.35
Generation of formaldehyde gas from solid polymer.
Mode of Action: See 1.1, 1.2, 1.3.
1.3.4.5
Formaldehyde Steam
Low-temperature steam with formaldehyde is used as a low-temperature sterilization
method in many countries, particularly in Scandinavia, Germany, and the United Kingdom.
The process involves the use of formalin (aqueous solution of formaldehyde, i.e. 37%
by weight), which is vaporized into a formaldehyde gas that is admitted into the sterilization
chamber. A formaldehyde concentration of 8–16 mg/l is generated at an operating temperature
of 70–75 °C. The sterilization cycle consists of a series of stages:
1.
Initial vacuum to remove air from the chamber and load
2.
Steam admission to the chamber with the vacuum pump running to purge the chamber of
air and to heat the load
3.
Followed by a series of pulses of formaldehyde gas
4.
Followed by steam.
5.
Formaldehyde is removed from the sterilizer and load by repeated alternate evacuations
and flushing with steam and air.
Mode of Action: See 1.1, 1.2, 1.3, 1.4
Advantages:
•
Cycle time for formaldehyde gas is faster than that for EtO
•
The cost per cycle is relatively low.
Disadvantages:
•
EtO is more penetrating and operates at lower temperatures than do steam/formaldehyde
sterilizers
•
Formaldehyde is a mutagen and a potential human carcinogen, and OSHA regulates formaldehyde
•
The formaldehyde steam sterilization system has not been FDA cleared for use in health
care facilities.
1.3.4.6
Ozone
Ozone has been used for years as a drinking water disinfectant. Ozone is produced
when oxygen is energized and split into two monatomic molecules. The monatomic oxygen
molecules then collide with O2 molecules to form ozone, which is O3. The additional
oxygen atom makes ozone a powerful oxidant that destroys microorganisms but is highly
unstable (i.e. half-life of 22 min at room temperature).
A new sterilization process, which uses ozone as the sterilant, was cleared by FDA
in August 2003 for processing reusable medical devices. The sterilizer, introduced
by TSO3 Inc. (Quebec City, QC, Canada), creates its own sterilant internally from
USP-grade oxygen, steam-quality water, and electricity; the sterilant is converted
back to oxygen and water vapor at the end of the cycle by a passing through a catalyst
before being exhausted into the room.
The ozone sterilization process uses two identical half-cycles. After the chamber
is loaded with instruments, the door is closed, and the cycle begins.
1.
A vacuum is created within the chamber
2.
Humidification
3.
Ozone is then injected into the chamber
4.
The previous steps are repeated
5.
A final ventilation phase is used to remove ozone from the chamber and the packaging
within it, the residual ozone is catalytically converted to oxygen.
The duration of the sterilization cycle is about 4 h and 15 min, and it occurs at
30–35 °C.
Advantages:
•
Claimed cost per cycle is very low
•
Employee safety
•
Can used sterilized items immediately.
Disadvantages:
•
Several metals may not be used.
Combined hydrogen peroxide and ozone systems have been introduced.
1.3.4.7
Gaseous Chlorine Dioxide
A gaseous chlorine dioxide system for sterilization of health care products was developed
in the late 1980s. Chlorine dioxide is not mutagenic or carcinogenic in humans. The
process is:
1.
Precondition: Raising of relative humidity levels to between 60% and 75%. Increased
humidity levels are necessary for all spore reduction.
2.
Condition: Hold time once relative humidity set point has been reached. This is to
allow spores to swell and crack due to humidity prior to gas introduction.
3.
Charge: Generation and delivery of chlorine dioxide gas.
4.
Exposure: Hold time once chlorine dioxide gas concentration set point has been reached.
5.
Aeration: Removal of chlorine dioxide gas from chamber.
Mode of Action: Chlorine dioxide (ClO2) acts as an oxidizing agent and reacts with
several cellular constituents, including the cell membrane and proteins of microbes.
1.3.4.8
Vaporized Peracetic Acid
The sporicidal activity of peracetic acid vapor at 20%, 40%, 60%, and 80% relative
humidity and 25 °C was determined on Bacillus atrophaeus spores on paper and glass
surfaces. Appreciable activity occurred within 10 min of exposure to 1 mg of peracetic
acid per liter at 40% or higher relative humidity. No vaporized peracetic acid system
is FDA cleared.
1.3.5
Ionizing Radiation
Sterilization by ionizing radiation, primarily by Cobalt-60 gamma rays or electron
accelerators, is a low-temperature sterilization method that has been used for a number
of medical products (e.g. tissue for transplantation, pharmaceuticals, and medical
devices). Gamma and electron beam (or beta) radiation is discussed in detail in the
earlier sections on the irradiation of food. The radiation process is not any different
for medical items, but in general only disposable or one-time use items are sterilized
by these methods because the facilities large and expensive and are not located on
hospital grounds. Major advantages of gamma and electron beam irradiation are that
there are no residuals and no radioactivity remaining. As soon as the delivered dose
of radiation is verified, products may be released for shipment.
There are no FDA-cleared ionizing radiation sterilization processes for use in health
care facilities. Because of high sterilization costs, this method is an unfavorable
alternative to EtO and plasma sterilization in health care facilities but is suitable
for large-scale sterilization.
1.3.5.1
UV Radiation
The wavelength of UV radiation ranges from 328 nm to 210 nm (3280 Å to 2100 Å). Its
maximum bactericidal effect occurs at 240–280 nm. Mercury vapor lamps emit more than
90% of their radiation at 253.7 nm, which is near the maximum microbicidal activity.
Inactivation of microorganisms results from destruction of nucleic acid through induction
of thymine dimers. This is discussed in 1.1, 1.2. UV radiation has been employed in
the disinfection of drinking water, air, titanium implants, and contact lenses. Bacteria
and viruses are more easily killed by UV light than are endospores.
The application of UV radiation in the health care environment (i.e. operating rooms,
isolation rooms, and biologic safety cabinets) is limited to destruction of airborne
organisms or inactivation of microorganisms on surfaces. There are two examples of
airborne use of UV. Figure 1.36
shows that UV lights may be mounted within air ducts to destroy airborne organisms;
such an application might be found in operating room ventilation. Figure 1.37
shows an example of UV use in an isolation room. Table 1.9
shows the UV dose required to reduce the populations of various organisms.
Figure 1.36
Diagram of ductwork-installed ultraviolet germicidal irradiation.
22
For color version of this figure, the reader is referred to the online version of
this book.
Figure 1.37
Diagram of hospital bed air space ultraviolet germicidal irradiation.
22
For color version of this figure, the reader is referred to the online version of
this book.
Table 1.9
UV Dose Required to Reduce the Population of Various Microorganisms
23
Organisms
Energy Dosage of Ultraviolet Radiation (UV Dose) in μWs/cm2 Needed for Kill Factor
Bacteria
90% Reduction
99% Reduction
Bacillus anthracis – anthrax
4520
8700
Bacillus anthracis spores – anthrax spores
24,320
46,200
Bacillus megaterium sp. (spores)
2730
5200
Bacillus megaterium species (veg.)
1300
2500
Bacillus paratyphus
3200
6100
Bacillus subtilis spores
11,600
22,000
Bacillus subtilis
5800
11,000
Clostridium tetani
13,000
22,000
Corynebacterium diphtheriae
3370
6510
Eberthella typhosa
2,140
4100
Escherichia coli
3000
6600
Leptospira canicola – infectious jaundice
3150
6000
Micrococcus candidus
6050
12,300
Micrococcus sphaeroides
1000
15,400
Mycobacterium tuberculosis
6200
10,000
Neisseria catarrhalis
4400
8500
Phytomonas tumefaciens
4400
8000
Proteus vulgaris
3000
6600
Pseudomonas aeruginosa
5500
10,500
Pseudomonas fluorescens
3500
6600
Salmonella enteritidis
4000
7600
Salmonella paratyphi – enteric fever
3200
6100
Salmonella typhosa – typhoid fever
2150
4100
Salmonella typhimurium
8000
15,200
Sarcina lutea
19,700
26,400
Serratia marcescens
2420
6160
Shigella dysenteriae – dysentery
2200
4200
Shigella flexneri – dysentery
1700
3400
Shigella paradysenteriae
1680
3400
Spirillum rubrum
4400
6160
Staphylococcus albus
1840
5720
Staphylococcus aureus
2600
6600
Staphylococcus hemolyticus
2160
5500
Staphylococcus lactis
6150
8800
Streptococcus viridans
2000
3800
Vibrio cholerae
3375
6500
Molds
90%
99%
Aspergillus flavus
60,000
99,000
Aspergillus glaucus
44,000
88,000
Aspergillus niger
132,000
330,000
Mucor racemosus A
17,000
35,200
Mucor racemosus B
17,000
35,200
Oospora lactis
5000
11,000
Penicillium expansum
13,000
22,000
Penicillium roqueforti
13,000
26,400
Penicillium digitatum
44,000
88,000
Rhizopus nigricans
111,000
220,000
Protozoa
90%
99%
Paramecium
11,000
20,000
Algae
Chlorella vulgaris
13,000
22,000
Helminthes
Nematode eggs
45,000
92,000
Virus
90%
99%
Bacteriophage – Escherichia coli
2600
6600
Infectious hepatitis A and E
5800
8000
Influenza
3400
6600
Poliovirus – Poliomyelitis
3150
6600
Tobacco mosaic
240,000
440,000
Yeast
90%
99%
Brewers yeast
3300
6600
Common yeast cake
6000
13,200
Saccharomyces cerevisiae
6000
13,200
Saccharomyces ellipsoideus
6000
13,200
Saccharomyces spores
8000
17,600
1.4
Bioterrorism
Bioterrorism is also a constant threat that the public really learned about in September
of 2001, when anthrax spores were intentionally sent through the postal system, causing
22 cases of anthrax, including 5 deaths, and forever changing the realm of public
health.
24
Mail to government offices was irradiated to prevent anthrax infections and contaminated
rooms and buildings were fumigated by methods discussed in this chapter.
The principal means of decontamination of facilities experiencing an anthrax attack
is fumigation with chlorine dioxide gas. Delivery of anthrax has been by mail in the
past. Irradiation of mail is an effort to disinfect it. The most notable instance
of mail irradiation occurred in response to the 2001 anthrax attacks; the level of
radiation chosen to kill anthrax spores was so high that it often changed the physical
appearance of the mail. A facility in Bridgeport, NJ, operated by Sterigenics International,
uses a Rhodotron continuous wave electron beam accelerator built by IBA Industrial
to irradiate the mail. A few facilities were planning to use Cobalt-60 sources, though
it is unclear whether this was ever done. During irradiation, an intense stream of
electrons (or X-rays if X-ray technology is used) strikes the mail and any anthrax
spores it may contain. The radiation dose is very high, about 56 kGy of radiation,
which is approximately 2 million times more than a chest X-ray.
List of Potential Bioterrorism Agents
25
CDC Category A Agents
Highest priority agents: Organisms that pose a risk to national security because:
○
They can be easily disseminated or transmitted from person to person
○
They result in high mortality rates and have the potential for major public health
impact
○
They might cause public panic and social disruption
○
They require special action for public health preparedness.
•
Anthrax (Bacillus anthracis)
•
Botulism (Clostridium botulinum toxin)
•
Plague (Yersinia pestis)
•
Smallpox (Variola virus)
•
Tularemia (Franciscella tularensis)
•
Viral hemorrhagic fever (Arenaviruses, Bunyaviruses).
CDC Category B Agents
Second highest priority agents:
○
Organisms that are moderately easy to disseminate
○
Result in moderate morbidity rates and low mortality rates
○
Require enhanced diagnostic capacity and disease surveillance.
•
Brucellosis (Brucella species)
•
Epsilon toxin of Clostridium perfringens
•
Food safety threats (Salmonella species, Escherichia coli O157:H7, Shigella)
•
Glanders (Burkholderia mallei)
•
Melioidosis (Burkholderia pseudomallei)
•
Psittacosis (Chlamydia psittaci)
•
Q fever (Coxiella burnetii)
•
Ricin toxin from Ricinus communis (castor beans)
•
Staphylococcal enterotoxin B
•
Typhus fever (Rickettsia prowazekii)
•
Viral encephalitis (e.g. Venezuelan equine encephalitis, eastern equine encephalitis,
western equine encephalitis)
•
Water safety threats (e.g. Vibrio cholerae, Cryptosporidium parvum).
CDC Category C Agents
These third highest priority agents include emerging pathogens that could be engineered
for mass spread in the future because:
○
They are easily available
○
They are easily produced and spread
○
They have potential for high morbidity and mortality rates and major health impact.
•
Nipah virus
•
Hantavirus
•
Severe acute respiratory syndrome virus
•
H1N1, a strain of influenza (flu)
•
HIV/AIDS.
1.5
Summary
This chapter reviewed briefly dozens of sterilization methods. The effect of sterilization
processes on plastic materials is important because most food and many medical supplies
are already packaged before being processed for microbe elimination. Packaging is
usually made from polymeric plastics. If the sterilization processes weaken the plastic
films, they could affect shelf life and damage losses.
The effect of all these methods on plastics is not covered in the data chapters. This
is because the effect of many is minimal on the plastic properties. The most commonly
used processes get the focus in the data chapters. Appendix A, Guidelines for Component
Sterilization based on Material and Process, does contain guidelines on the general
suitability of many of the processes on various plastics.
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Formaldehyde, Formalin, Paraformaldehyde And Glutaraldehyde: What They Are And What They Do
John Kiernan (2000)
- Record: found
- Abstract: found
- Article: not found
New disinfection and sterilization methods.
W A Rutala, D J Weber (2001)
- Record: found
- Abstract: found
- Article: not found
Mechanism of microwave sterilization in the dry state.
Leonard Kaczmarek, Bradford Woodworth, Emily K Jeng … (1987)