- Record: found
- Abstract: found
- Article: not found
The Rab5 Effector Rabankyrin-5 Regulates and Coordinates Different Endocytic Mechanisms
Read this article at
Abstract
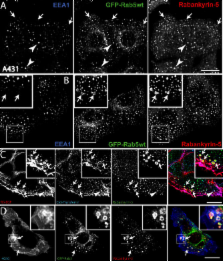
The small GTPase Rab5 is a key regulator of clathrin-mediated endocytosis. On early endosomes, within a spatially restricted domain enriched in phosphatydilinositol-3-phosphate [PI(3)P], Rab5 coordinates a complex network of effectors that functionally cooperate in membrane tethering, fusion, and organelle motility. Here we discovered a novel PI(3)P-binding Rab5 effector, Rabankyrin-5, which localises to early endosomes and stimulates their fusion activity . In addition to early endosomes, however, Rabankyrin-5 localises to large vacuolar structures that correspond to macropinosomes in epithelial cells and fibroblasts. Overexpression of Rabankyrin-5 increases the number of macropinosomes and stimulates fluid-phase uptake, whereas its downregulation inhibits these processes. In polarised epithelial cells, this function is primarily restricted to the apical membrane. Rabankyrin-5 localises to large pinocytic structures underneath the apical surface of kidney proximal tubule cells, and its overexpression in polarised Madin-Darby canine kidney cells stimulates apical but not basolateral, non-clathrin-mediated pinocytosis. In demonstrating a regulatory role in endosome fusion and (macro)pinocytosis, our studies suggest that Rab5 regulates and coordinates different endocytic mechanisms through its effector Rabankyrin-5. Furthermore, its active role in apical pinocytosis in epithelial cells suggests an important function of Rabankyrin-5 in the physiology of polarised cells.
Abstract
Rabankyrin-5, which is activated by the small GTPase Rab5, coordinates two disparate methods that cells use to take in solids and fluids, playing a key role in both endosome fusion and macropinocytosis
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
Regulated portals of entry into the cell.
- Record: found
- Abstract: found
- Article: not found
A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages
- Record: found
- Abstract: found
- Article: not found
