- Record: found
- Abstract: found
- Article: found
Tertiary lymphoid structures in cancer: immune mechanisms and clinical implications
Read this article at
Abstract
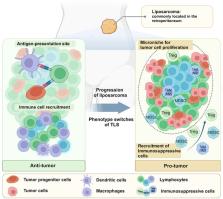
Cancer is a major cause of death globally, and traditional treatments often have limited efficacy and adverse effects. Immunotherapy has shown promise in various malignancies but is less effective in tumors with low immunogenicity or immunosuppressive microenvironment, especially sarcomas. Tertiary lymphoid structures (TLSs) have been associated with a favorable response to immunotherapy and improved survival in cancer patients. However, the immunological mechanisms and clinical significance of TLS in malignant tumors are not fully understood. In this review, we elucidate the composition, neogenesis, and immune characteristics of TLS in tumors, as well as the inflammatory response in cancer development. An in‐depth discussion of the unique immune characteristics of TLSs in lung cancer, breast cancer, melanoma, and soft tissue sarcomas will be presented. Additionally, the therapeutic implications of TLS, including its role as a marker of therapeutic response and prognosis, and strategies to promote TLS formation and maturation will be explored. Overall, we aim to provide a comprehensive understanding of the role of TLS in the tumor immune microenvironment and suggest potential interventions for cancer treatment.
Abstract
Tertiary lymphoid structures (TLSs) are unencapsulated aggregates of organized lymphocytes in chronic inflammatory or tumor tissues, which has been detected in a series of malignant tumors such as lung cancer, breast cancer, melanoma, virous sarcomas and so on. Previous studies have indicated that TLS could have significant clinical implications, including being a commonly favorable prognostic marker. However, these reports are usually inconsistent in different research, especially in sarcomas. Soft tissue sarcomas have extensive immunologic heterogeneity, including genetic alterations, expression of neoantigens, and transcription of immune checkpoint molecules. These immune characteristics lead to unsatisfactory immunotherapy effect and more side effects. Therefore, improved treatment strategies based on the immune characteristics of sarcoma need to be developed. The generation of tumor‐localized TLS can be facilitated by a series of therapeutic modalities, which could potentially mitigate the immunosuppressive phenotype of sarcoma and enhance treatment response. This strategy can also be extended to other malignancies as well. In this comprehensive review, we elucidate the formation mechanisms and composition of tumor‐associated TLS and provide an in‐depth discussion of immune mechanisms and clinical implications of TLS in three common cancers and soft tissue sarcomas. Combined with the existing treatment strategies, we propose possible intervention programs centered around TLS modulation, which provides a new evaluation index and treatment strategy for the treatment of cancer.
Related collections
Most cited references235
- Record: found
- Abstract: found
- Article: not found
Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.
- Record: found
- Abstract: found
- Article: not found
Fundamental Mechanisms of Immune Checkpoint Blockade Therapy
- Record: found
- Abstract: found
- Article: not found
