- Record: found
- Abstract: found
- Article: found
LINC00540 promotes sorafenib resistance and functions as a ceRNA for miR-4677-3p to regulate AKR1C2 in hepatocellular carcinoma
Read this article at
Abstract
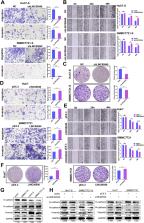
Sorafenib resistance is one of the main causes of poor prognosis in patients with advanced hepatocellular carcinoma (HCC). Long noncoding RNAs (lncRNAs) function as suppressors or oncogenic factors during tumor progression and drug resistance. Here, to identify therapeutic targets for HCC, the biological mechanisms of abnormally expressed lncRNAs were examined in sorafenib-resistant HCC cells. Specifically, we established sorafenib-resistant HCC cell lines (Huh7-S and SMMC7721-S), which displayed an epithelial-mesenchymal transition (EMT) phenotype. Transcriptome sequencing (RNA-Seq) was performed to established differential lncRNA expression profiles for sorafenib-resistant cells. Through this analysis, we identified LINC00540 as significantly up-regulated in sorafenib-resistant cells and a candidate lncRNA for further mechanistic investigation. Functionally, LINC00540 knockdown promoted sorafenib sensitivity and suppressed migration, invasion, EMT and the activation of PI3K/AKT signaling pathway in sorafenib-resistant HCC cells, whereas overexpression of LINC00540 resulted in the opposite effects in parental cells. LINC00540 functions as a competing endogenous RNA (ceRNA) by competitively binding to miR-4677-3p , thereby promoting AKR1C2 expression. This is the first study that demonstrates a role for LINC00540 in enhancing sorafenib resistance, migration and invasion of HCC cells through the LINC00540/miR-4677-3p/AKR1C2 axis, suggesting that LINC00540 may represent a potential therapeutic target and prognosis biomarker for HCC.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found