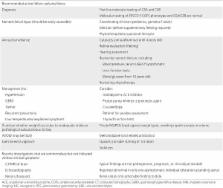
Neurodegeneration and cachectic dwarfism are cardinal symptoms of the autosomal recessive segmental progeria Cockayne syndrome (CS; Nance and Berry, 1992). Average life expectancy is 12 yr; however, the phenotype shows substantial variation in severity. 80% of CS cases are caused by mutations in the group B gene (CSB), with the remaining 20% caused by mutations in CSA. CSB is involved in transcription-coupled nucleotide excision DNA repair (TC-NER; Anindya et al., 2010) and has been proposed to carry out chromatin remodeling (Citterio et al., 2000), act as a transcription factor (Le May et al., 2010), and function as a co-regulator of base excision repair (BER; Stevnsner et al., 2008). The CSBm/m mouse displays a mild neurological phenotype, with an increased susceptibility to UV-induced cancer (van der Horst et al., 1997). This hypersensitivity to UV is a hallmark of CSB-deficient cells in culture and is related to the lack of TC-NER. However, CSB-deficient cells are also sensitive to alkylating and oxidizing agents (Stevnsner et al., 2008) and display defective repair of the DNA lesions 8-oxoguanine (Dianov et al., 1999) and 8-hydroxyadenine (Tuo et al., 2002). Oxidative lesions, repaired by BER, are particularly pertinent because the accumulation of oxidized proteins, lipids, and/or nucleic acids have been proposed to be an underlying cause of aging (Balaban et al., 2005). Endogenous oxidizing radicals originate predominantly from the mitochondria, where the mitochondrial BER machinery acts as the primary protector. Recent findings indicate that CSB is present in the mitochondria, suggesting a role for CSB in mitochondrial DNA (mtDNA) repair (Aamann et al., 2010; Kamenisch et al., 2010). We now report that CSB participates in mitochondrial maintenance by inducing autophagy in response to mitochondrial stress. Lack of CSB leads to mitochondrial dysfunction and increased metabolism, both at the organismal and cellular level. Accordingly, we show that pharmacological activation of autophagy reverses the bioenergetic profile. Based on this, we propose that CSB acts as a sensor of mtDNA damage and regulates mitochondrial autophagy and that treatment with rapamycin or lithium chloride could potentially attenuate some symptoms related to CS. RESULTS CSBm/m mice show age-related and universal loss of fat Because mitochondrial dysfunction can have an effect on overall metabolism, we investigated the distribution and volume of adipose tissue, using T1-weighted MRI, in old and young WT and CSBm/m mice. The combined volume of the abdominal and epididymal fat depots in old CSBm/m mice was only 36% of the amount in old WT mice (Fig. 1 A). Interestingly, this difference was already present in the young group, which showed 82% of the amount of fat compared with age-matched controls. The loss of fat was not only subcutaneous but also visceral fat (Fig. 1 B and Video 1). Curiously, during the MRI scans the old CSBm/m mice were found to be particularly difficult to maintain at a constant respiration frequency under anesthesia, as evident from the large fluctuations in respiration frequency (Fig. 1 C). Figure 1. The old CSBm/m mice show signs of neurodegeneration and universal loss of fat. (A) Quantification of adipose tissue using T1-weighted MRI in young (2 mo) and old (20 mo) WT and CSBm/m mice (n = 3; data are represented as mean ± SEM). (B) Representative axial T1-weighted MRI scans of the CSBm/m and WT mice. Yellow denotes fat. (C) Respiration rates of mice during the MRI scans. (D) HE staining of abdominal skin in young and old WT and CSBm/m mice. The bar denotes the subcutaneous fat layer (n = 3). (E) HE staining of the liver. (F) Periodic acid-Schiff staining of the liver of the 20-mo-old mice with and without diastase to show glycogen content. (G) HE staining of the inner ear. The star denotes the spiral ganglion (n = 3). (H) Brain weight of WT and CSBm/m mice (n = 3; data are represented as mean ± SEM). Skin histology of old CSBm/m mice also showed loss of subcutaneous fat by HE staining (Fig. 1 D) as previously reported (Kamenisch et al., 2010). Liver histology from the old WT mice, but not CSBm/m animals, showed vacuolization of the cytoplasm (Fig. 1 E) caused either by an accumulation of lipids or glycogen. Using periodic acid-Schiff staining, we found no difference in the amount of glycogen in the liver tissue (Fig. 1 F), indicating a decrease in liver lipids in old CSBm/m. The CSBm/m mouse shows a CS-like phenotype To further investigate a possible phenotype in the CSB-deficient mice, we performed extensive histology, hematology, and serology on young and old CSBm/m and WT mice. Table S1 shows a list of serum chemistry, hematology, and organ weights examined. The only overall significant difference uncovered when comparing the young WT and CSBm/m mice was an ∼50% increase in thymus weight (31 mg, WT vs. 48 mg, CSBm/m; P = 0.03), indicating a delay in organ involution; however, no histological disparity was observed (unpublished data). For the old CSBm/m mice, subtle signs of neurodegeneration were present. A substantial loss of spiral gangliocytes was found in the inner ear of the old CSBm/m mice (Fig. 1 G), consistent with the sensorineural hearing loss associated with CS (Nance and Berry, 1992). The CSBm/m mice displayed significantly smaller brain weights compared with WT (Fig. 1 H), although the cerebrum, cerebellum, spinal cord, and ischiatic nerve showed no sign of pathology (unpublished data). Several mitochondrial diseases show skeletal or heart myopathy, yet no histological changes were found in the CSBm/m mice (unpublished data), despite a trend toward increased plasma creatine kinase in the CSB-deficient mice (Table S1). CSBm/m mice show increased metabolism The observed fat loss could be the result of a decrease in food consumption, gastrointestinal malabsorption, or increased energy expenditure. Surprisingly, the old CSBm/m mice consumed ∼33% more food than the age-matched WT mice (Fig. 2 A, n = 3). No steatorrhea was observed, and histology of the jejunum, ileum, and colon did not reveal pathology that could indicate malabsorption in the mutant animals (Fig. 2, B and C). We therefore investigated the overall metabolism of the mice. Housing the animals in metabolic cages revealed increased O2 consumption and CO2 production without an increase in movement in the old CSBm/m mice (Fig. 2 D; movement data not depicted). Interestingly, there was a trend toward increased respiratory exchange rates in the old CSBm/m mice, indicating that the loss of fat was not the result of a preference for β-oxidation (Fig. 2 D). Serum chemistry showed no difference in blood glucose or cholesterol (Table S1); however, a decrease in circulating triglycerides was observed in old CSBm/m mice, reflecting the increased metabolism. Figure 2. CSB deficiency increases whole body metabolism. (A) 1 wk food intake CSBm/m compared with age-matched WT mice (n = 3; data are represented as mean ± SEM). (B and C) HE staining of the small (B) and large (C) intestines (n = 3). (D) Mice were placed in metabolic cages for 72 h. O2 consumption and CO2 production were measured. D1= dark phase 1, L1 = light phase 1, etc. (n = 3–8; data are represented as mean ± SEM; *, P 100). Lentivirus generation and infections. shRNA vectors against CSB were purchased from Santa Cruz Biotechnology, Inc. A cocktail of three vectors containing the following shRNA sequences were used: 5′-GTCTTACGAGATACCATAATTCAAGAGATTATGGTATCTCGTAAGACTTTTT-3′, 5′-CCAGAAGCAAGACAGTGAATTCAAGAGATTCACTGTCTTGCTTCTGGTTTTT-3′, and 5′-GTCTTCCGAGAACTATTGATTCAAGAGATCAATAGTTCTCGGAAGACTTTTT-3′. As a control, a plasmid containing a scrambled shRNA was used (Plasmid-A; Santa Cruz Biotechnology, Inc.). Lentiviral production was done by co-transfection of pCMV delta R8.2 (plasmid 12263 deposited by Didier Trono, Addgene Inc.) backbone, pCMV-VSV-G (plasmid 8454, deposited by Robert Weinberg, Addgene Inc.), and the vectors expressing the shRNA into 293T cells by Fugene 6 transfection as per manufacturer’s protocol (Roche). The media containing the virus was harvested after 48 h, filtered through 0.45-µm pore filter, flash frozen, and stored at −80°C. Cells were seeded at a density of 2 × 105 in 10-cm plates, 24 h before the infection. For infection, the media was removed and the cells were washed once with PBS. The frozen virus was thawed quickly and added to the plates. The plates were rocked every 15 min by hand and incubated at 37°C for 2 h. Fresh media was subsequently added. Cellular oxygen consumption. Oxygen consumption and extracellular acidification rate measurements were performed using the Seahorse XF-24 instrument (Seahorse Biosciences). Cells were seeded into a Seahorse tissue culture plate at a density according to Table S1, and 16 h later media was changed to unbuffered XF assay media at pH 7.4 (Seahorse Biosciences), supplemented with 25 mM glucose (Sigma-Aldrich), 1 mM sodium-pyruvate, and 1 mM glutamax (Invitrogen). Cells were incubated for 1 h at 37°C at ambient O2 and CO2 concentration before measurements were taken. Respiration was measured in four blocks of 3 × 3 min. The first block measured the basal respiration rate. Next, oligomycin (EMD) was added to inhibit complex V and the second block was measured. Then, FCCP (Sigma-Aldrich) was added to uncouple respiration and the third block was measured. Finally, antimycin A (Sigma-Aldrich) was added to inhibit complex 3 and the last measurements were performed. See Table S2 for compound concentrations used. All compound concentrations used had been optimized for that cell line. Immediately after finishing the measurements, cells were trypsinized and counted using a Coulter counter (Beckman Coulter). For HSMM, cells were plated in Seahorse tissue culture plates and allowed to grow to 90% confluency. The media was then changed to DME:F-12 (Lonza) supplemented with 3% fetal horse serum and the myoblasts were allowed to differentiate for 3 d. Lentiviral knockdown was then performed by addition of virus directly to each well. 3 d later, respiratory measurements were performed as described. After the experiment, myotubes were lysed in LB, the protein content was measured, and respiration was normalized to protein concentration. Lysates were subsequently used for immunoblotting to investigate knockdown efficiency. Mitochondrial membrane potential. In brief, 0.4 × 106 CS1AN cells expressing WT CSB or empty vector were harvested by trypsin, washed, and resuspended in DME without phenol indicator (Invitrogen). Cells were then stained with 20 nM TMRM for 15 min at 37°C with or without 1 µM cyclosporin A. Fluorescence was measured by a flow cytometer (C6; Accuri). Treatments with 10 mM lithium chloride (Sigma-Aldrich) or 1 µM rapamycin (Sigma-Aldrich) were done for 24 h before membrane potential. Glucose consumption. Glucose consumption was measured by plating 50,000 CS1AN cells expressing WT CSB or an empty vector in a 24-well dish (n = 3). 24 h later, media was changed to DME containing 10 mM glucose and supplemented with 1% pen-strep, 10% FBS, and 400 µg/ml geneticin. At the given time points, the glucose concentration was measured in the media using the GlucCell glucose monitoring system as per manufacturer’s instruction (Cesco Bioengineering). The cells were washed twice in PBS, trypsinized, and counted. ATP concentration. In brief, 10,000 cells per well were plated in a 96-well dish. 24 h later, ATP levels were measured in using the ATPlite assay according to manufacturer’s protocol (PerkinElmer). For the ATP consumption assay, 100 mM 2-deoxyglucose and 1 µM oligomycin was added at time 0. Cells were subsequently lysed at the indicated time points and the ATP concentration was measured. Luminescence was measured on the 1450 MicroBeta TriLux Microplate Scintillation and Luminescence Counter (PerkinElmer). Mitochondrial content. Citrate synthase activity (n = 3) was measured spectrophotometrically as described in Srere (1969) with modifications. Two different dilutions of the sample were loaded in duplicates into a 96-well dish. The linear increase in absorbance was measured at 412 nm over 5 min and the concentration was determined against a standard curve of purified citrate synthase (Sigma-Aldrich). Transfection with RFP-tagged mitochondrial protein (Organelle Lights; Invitrogen) was further used to investigate the amount of mitochondria according to manufacturer’s protocol (n = 3). In brief, 105 cells were plated in glass-bottom 20-mm plates. The next day, cells were washed in PBS and 500 µl of the transduction solution was added. Cells were incubated 2 h in 37°C before the transduction solution was removed and the enhancer solution was added. After 2 h of incubation at 37°C, the enhancer solution was removed and normal growth media was added. 24 h later, live cells were imaged at 40× on a confocal microscope (Eclipse TE-2000e; Nikon), with a 60 ms brightfield and 200 ms rfp exposure time. 0.2 µm z-stacks were used to image entire cells and, with an average of at least 25 cells per transfection, were used per replica experiment. Quantification was performed by normalizing voxel count to cell number using the Volocity software (PerkinElmer). Mitochondrial content was additionally investigated using MitoTracker green FM (Invitrogen) as previously described (Pendergrass et al., 2004) with slight modification (n = 3). In brief, 0.75 × 106 CS1AN cells expressing WT CSB or empty vector were harvested by trypsin, washed, and resuspended in DME without phenol indicator (Invitrogen). Half the cells were then stained with 50 nM MitoTracker green FM for 30 min at 37°C. Cells were washed twice and resuspended in PBS before fluorescence was measured by a flow cytometer (C6). Unstained cells were used to gate for stained cells. PCR. Total RNA was isolated from cells using Trizol reagent (Invitrogen) per the manufacturer’s protocol (n = 3). Reverse transcription reactions were performed using Iscript (Bio-Rad Laboratories). Primers were designed using the Primer Express 2.0 (Applied Biosystems). The sequences of primers are shown in Table S3. Real-time PCR was performed using SYBR green PCR mix (Applied Biosystems) with a primer concentration of 500 nM. Hypoxanthine phosphoribosyltransferase (HPRT) served as an internal control to normalize samples. Western blots. Western blots were performed according to standard procedure. 30–100 µg proteins per lane were used. Samples were analyzed using 4–20% tris-glycine SDS-PAGE (Invitrogen). The following antibodies were used: rabbit anti-CSB (Santa Cruz Biotechnology, Inc.), rabbit anti-LC3B (Novus Biologicals), mouse anti-GAPDH (Santa Cruz Biotechnology, Inc.), rabbit anti-lamin A/C (Santa Cruz Biotechnology, Inc.), mouse anti-P62 (Abcam), goat anti-COX4 (Santa Cruz Biotechnology, Inc.), and rabbit anti-ubiquitin (Santa Cruz Biotechnology, Inc.). Secondary HRP-conjugated antibodies and ECL plus (GE Healthcare) were used to visualize the protein bands on a Typhoon scanner. Quantification was performed using ImageQuant (GE Healthcare). For autophagy, 1 × 106 CS1AN cells were plated in 100 mm dishes (n = 3). 24 h later, cells were treated with vehicle, 5 µM rotenone, or 500 ng/ml ethidium bromide. Cells were incubated 24 h and harvested. Cells were lysed in LB. For HSMM, 10 × 105 myoblasts were plated in 100 mm dishes and differentiated as described. Lentiviral knockdown was performed and, 3 d later, cells were treated with vehicle, 5 µM rotenone, or 500 ng/ml ethidium bromide before they were harvested. Microscopy. To investigate colocalization, 5 ×104 cells were plated in 4-well chamber slides and grown overnight. The next day, the media was changed and vehicle, 5 µM rotenone, or 100 nM bafilomycin was added. Cells were treated for 24 h before being fixed for 15 min in 3.7% paraformaldehyde in PBS. Cells were then washed before being permeabilized in 0.25% Triton X-100 for 10 min on ice. Subsequently, cells were washed in PBS and blocked overnight in PBS containing 5% FBS. Primary antibodies were added at a concentration of 1:100 and incubated for 1 h at 37°C. After being washed, secondary antibodies were added at 1:1,000 and incubated for 1 h at 37°C. Cells were then washed 6 × 10 min in PBS before being mounted in prolong antifade gold with DAPI (Invitrogen). Images were acquired at 60× on a confocal microscope (Eclipse TE-2000e), 50 ms brightfield, 300 ms exposure time for COX4 at 488 nm, and 900 ms exposure time for P62 or LC3B at 647 nm. Quantification was done by averaging the unbiased global Pearson’s coefficients of at least 10 unselected images per experiment using Volocity software. For live cell imaging of LC3B-eGFP, 105 cells were plated in glass-bottom 20-mm plates. The next day, cells were transfected with LC3-EGFP plasmid (plasmid 11546, deposited by K. Kirkegaard, Addgene Inc.) using Fugene-6 transfection according to the manufacturer’s protocol (Roche). 2 d later, live cell imaging was performed at 40× on a confocal microscope (Eclipse TE-2000e), with a 60 ms brightfield and 600 ms gfp exposure time. 0.2 µm z-stacks were used to image entire cells, and the means of at least 20 cells per transfection were used per replica experiment (n = 3). Quantification was performed by automatic unbiased spot finding using the Volocity software. ROS production. The ROS marker dihydroethidium (DHE; Invitrogen) was used to measure intracellular ROS, predominantly superoxide (n = 10). 4 µM DHE induced no visible cellular toxicity after 2 h of incubation and was subsequently used to measure ROS. Target cells were plated into 96-well plates 12 h before the experiment. Before commencing the assay, the medium was removed and cells washed twice with PBS before the addition of DHE probe diluted in prewarmed DME without phenol indicator (Invitrogen). Fluorescence was measured using 520 nm excitation/610 nm emission at 5-min intervals for 60 min using a FLUOstar Optima (BMG Labtech) with bottom reading capability. The mitochondrial ETC blocker antimycin A was used as a positive control and to ensure that cellular dye uptake was not rate limiting. After completion of the assay, the cells in each well were counted and used to normalize the results. Statistics. Prism 4.0 (GraphPad Software) was used to plot data, make graphs, and statistical analysis. Data are plotted as mean ± SEM. Unless otherwise stated, two-tailed unpaired Student’s t test was performed. Online supplemental material. Fig. S1 shows the list of genes significantly changed in the analyzed microarray as well as the quality control we performed on the data. Video 1 shows a representative axial MRI scan of the young and old WT and CSBm/m mice. Table S1 shows serum chemistry, hematology, and organ weights of the old and young WT and CSBm/m mice. Table S2 shows a list of cells and compound concentrations used in the oxygen consumption experiments. Table S3 shows a list of primers used for quantitative PCR. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20111721/DC1.