- Record: found
- Abstract: found
- Article: found
Applications of functionally-adapted hydrogels in tendon repair
Read this article at
Abstract
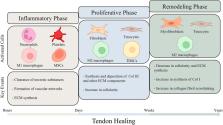
Despite all the efforts made in tissue engineering for tendon repair, the management of tendon injuries still poses a challenge, as current treatments are unable to restore the function of tendons following injuries. Hydrogels, due to their exceptional biocompatibility and plasticity, have been extensively applied and regarded as promising candidate biomaterials in tissue regeneration. Varieties of approaches have designed functionally-adapted hydrogels and combined hydrogels with other factors (e.g., bioactive molecules or drugs) or materials for the enhancement of tendon repair. This review first summarized the current state of knowledge on the mechanisms underlying the process of tendon healing. Afterward, we discussed novel strategies in fabricating hydrogels to overcome the issues frequently encountered during the applications in tendon repair, including poor mechanical properties and undesirable degradation. In addition, we comprehensively summarized the rational design of hydrogels for promoting stem-cell-based tendon tissue engineering via altering biophysical and biochemical factors. Finally, the role of macrophages in tendon repair and how they respond to immunomodulatory hydrogels were highlighted.
Related collections
Most cited references172
- Record: found
- Abstract: found
- Article: not found
Macrophage plasticity, polarization, and function in health and disease.
- Record: found
- Abstract: found
- Article: not found
Matrix elasticity directs stem cell lineage specification.
- Record: found
- Abstract: found
- Article: not found