- Record: found
- Abstract: found
- Article: found
Hemolytic Dynamics of Weekly Primaquine Antirelapse Therapy Among Cambodians With Acute Plasmodium vivax Malaria With or Without Glucose-6-Phosphate Dehydrogenase Deficiency
Read this article at
Abstract
Background
Hemoglobin (Hb) data are limited in Southeast Asian glucose-6-phosphate dehydrogenase (G6PD) deficient (G6PD −) patients treated weekly with the World Health Organization–recommended primaquine regimen (ie, 0.75 mg/kg/week for 8 weeks [PQ 0.75]).
Methods
We treated Cambodians who had acute Plasmodium vivax infection with PQ 0.75 and a 3-day course of dihydroartemisinin/piperaquine and determined the Hb level, reticulocyte count, G6PD genotype, and Hb type.
Results
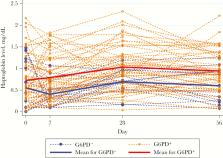
Seventy-five patients (male sex, 63) aged 5–63 years (median, 24 years) were enrolled. Eighteen were G6PD deficient (including 17 with G6PD Viangchan) and 57 were not G6PD deficient; 26 had HbE (of whom 25 were heterozygous), and 6 had α-/β-thalassemia. Mean Hb concentrations at baseline (ie, day 0) were similar between G6PD deficient and G6PD normal patients (12.9 g/dL [range, 9‒16.3 g/dL] and 13.26 g/dL [range, 9.6‒16 g/dL], respectively; P = .46). G6PD deficiency ( P = <.001), higher Hb concentration at baseline ( P = <.001), higher parasitemia level at baseline ( P = .02), and thalassemia ( P = .027) influenced the initial decrease in Hb level, calculated as the nadir level minus the baseline level (range, −5.8–0 g/dL; mean, −1.88 g/dL). By day 14, the mean difference from the day 7 level (calculated as the day 14 level minus the day 7 level) was 0.03 g/dL (range, −0.25‒0.32 g/dL). Reticulocyte counts decreased from days 1 to 3, peaking on day 7 (in the G6PD normal group) and day 14 (in the G6PD deficient group); reticulocytemia at baseline ( P = .001), G6PD deficiency ( P = <.001), and female sex ( P = .034) correlated with higher counts. One symptomatic, G6PD-deficient, anemic male patient was transfused on day 4.
Abstract
Among Plasmodium vivax–infected Cambodians with glucose-6-phopshate dehydrogenase (G6PD) deficiency who were treated weekly with primaquine, 6 had marked decreases in the hemoglobin (Hb) level, and 1 was transfused. G6PD deficiency and HbE were consistently important factors associated with changes in the Hb level over time.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: found
KDIGO Clinical Practice Guidelines for Acute Kidney Injury

- Record: found
- Abstract: found
- Article: found
Determinants of relapse periodicity in Plasmodium vivax malaria
- Record: found
- Abstract: found
- Article: not found