- Record: found
- Abstract: found
- Article: found
Multi-target mechanism of Naoshuantong capsule for treatment of Ischemic stroke based on network pharmacology and molecular docking
Read this article at
Abstract
Background:
Naoshuantong capsule (NST capsule) is a classic Chinese patent medicine, which can treat ischemic stroke (IS) and has good clinical efficacy. However, its pharmacological mechanism remains to be further explored in the treatment of IS.
Methods:
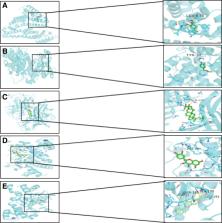
The bio-active components and potential targets of NST Capsules were obtained by ETCM and TCMSP databases. In addition, the related targets of IS were collected by Genecard, OMIM, DrugBank, TTD and DisGeNET databases. NST-IS common target was obtained by Venn platform. PPI network of NST-IS common target and the composition - target network diagram of NST Capsule were constructed by Cytoscape3.8.1. Finally, AutoDock was used for molecular docking.
Results:
265 targets were predicted from 32 active compounds in NST Capsule, 109 common targets were identified between NST Capsule and IS. The top 10 key targets of PPI network were ALB, TNF, TP53, VEGFA, CASP3, MYC, etc. Enrichment analysis showed that NST capsules treated IS mainly through lipid and atherosclerosis, fluid shear stress and atherosclerosis signaling pathways.
Conclusion:
Through the methods of network pharmacology and molecular docking, this study clarified that NST capsules play a role in the treatment of IS, which is multi-target, multi-channel and multi-component regulation. This study further explored the pharmacological mechanism of NST capsule in the treatment of IS, which can provide some references for the subsequent research in the pharmacological mechanism of NST capsule.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications

- Record: found
- Abstract: found
- Article: found
Modulators of microglia activation and polarization in ischemic stroke

- Record: found
- Abstract: found
- Article: found