- Record: found
- Abstract: found
- Article: found
Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds
Read this article at
Abstract
Bone-tissue defects affect millions of people worldwide. Despite being common treatment approaches, autologous and allogeneic bone grafting have not achieved the ideal therapeutic effect. This has prompted researchers to explore novel bone-regeneration methods. In recent decades, the development of bone tissue engineering (BTE) scaffolds has been leading the forefront of this field. As researchers have provided deep insights into bone physiology and the bone-healing mechanism, various biomimicking and bioinspired BTE scaffolds have been reported. Now it is necessary to review the progress of natural bone physiology and bone healing mechanism, which will provide more valuable enlightenments for researchers in this field. This work details the physiological microenvironment of the natural bone tissue, bone-healing process, and various biomolecules involved therein. Next, according to the bone physiological microenvironment and the delivery of bioactive factors based on the bone-healing mechanism, it elaborates the biomimetic design of a scaffold, highlighting the designing of BTE scaffolds according to bone biology and providing the rationale for designing next-generation BTE scaffolds that conform to natural bone healing and regeneration.
Graphical abstract
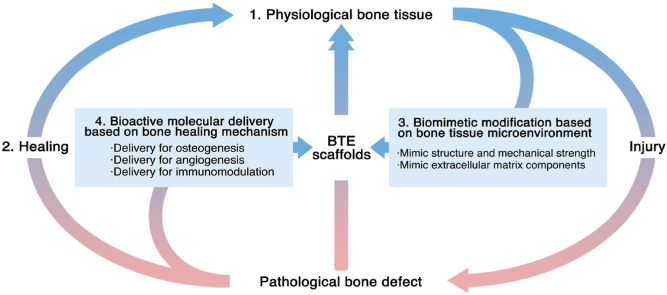
Bone-tissue engineering has become a promising treatment strategy for large bone defects. This work first introduces the advanced knowledge of bone biology, including the physiological microenvironment and healing process. Based on this concept, it further details the current biomimetic and bioactive bone-tissue engineering scaffolds promoting the healing process. Finally, it provides the future perspective in this field.
Highlights
-
•
Elaborate the advanced knowledge of bone physiological microenvironment and healing process.
-
•
Summary the biomolecules involved in the natural bone healing process which could be applied to BTE materials and scaffolds.
-
•
Detail the current biomimetic and bioinspired scaffolds based on the bone physiological microenvironment.
-
•
Review the delivery of bioactive factors based on the bone healing mechanism.
-
•
Discuss the current limitations that still need to be solved, and the feasible improvement and outlooks are proposed.
Related collections
Most cited references347
- Record: found
- Abstract: found
- Article: not found
Macrophage activation and polarization: nomenclature and experimental guidelines.
- Record: found
- Abstract: found
- Article: not found
Neutrophil recruitment and function in health and inflammation.
- Record: found
- Abstract: found
- Article: not found