- Record: found
- Abstract: found
- Article: found
Inhibition of protein tyrosine phosphatase improves mitochondrial bioenergetics and dynamics, reduces oxidative stress, and enhances adipogenic differentiation potential in metabolically impaired progenitor stem cells

Read this article at
Abstract
Background
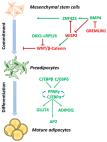
Protein tyrosine phosphatase 1B (PTP1B) and low molecular weight protein tyrosine phosphatase (LMPTP) are implicated in the development of metabolic disorders. Yet, their role in progenitor stem cell adipogenic differentiation and modulation of mitochondrial dynamics remains elusive.
Methods
In this study, we decided to investigate whether inhibition of PTP1B and LMPTP enhance adipogenic differentiation of metabolically impaired progenitor stem cells via modulation of mitochondrial bioenergetics and dynamics. Cells were cultured under adipogenic conditions in the presence of PTP1B and LMPTP inhibitors, and were subjected to the analysis of the main adipogenic-related and mitochondrial-related genes using RT-qPCR. Protein levels were established with western blot while mitochondrial morphology with MicroP software.
Results
Selective inhibitors of both PTP1B and MPTP enhanced adipogenic differentiation of metabolically impaired progenitor stem cells. We have observed enhanced expression of PPARy and adiponectin in treated cells. What is more, increased antioxidative defence and alternations in mitochondrial bioenergetics were observed. We have found that inhibition of PTP1B as well as C23 activates oxidative phosphorylation and enhances mitochondrial fusion contributing to enhanced adipogenesis.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: found
The Global Epidemic of the Metabolic Syndrome
- Record: found
- Abstract: found
- Article: found
Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications
- Record: found
- Abstract: found
- Article: not found
Targeting adipose tissue in the treatment of obesity-associated diabetes
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.
