- Record: found
- Abstract: found
- Article: found
老年肺癌患者的免疫治疗疗效分析 Translated title: Analysis of the Efficacy of Immunotherapy in Elderly Patients with Lung Cancer
Read this article at
Abstract
背景与目的
以免疫检查点抑制剂(immune checkpoint inhibitors, ICIs)为代表的免疫疗法成为驱动基因阴性晚期非小细胞肺癌(non-small cell lung cancer, NSCLC)的标准治疗方式。但是,肺癌高发于老年患者,而这部分患者很少被纳入重要的临床试验研究。我们旨在研究“真实世界”老年肺癌人群免疫治疗的疗效和安全性。
方法
回顾性分析2018年7月-2021年10月期间接受免疫治疗的老年NSCLC患者和同期的年轻患者,比较不同年龄分组(< 60岁组为中青年组,60岁-74岁为年轻老年组,75岁及以上为一般老年组)患者的客观缓解率(objective response rate, ORR)和无进展生存期(progression-free survival, PFS),并在各年龄亚组中分析不同临床特征对疗效的影响。
Translated abstract
Immunotherapy represented by immune checkpoint inhibitors (ICIs) has become the standard treatment for patients with non-oncogenic advanced non-small cell lung cancer (NSCLC). While lung cancer is most prevalent in elderly patients, these patients are rarely included in pivotal clinical trial studies. We aimed to describe the efficacy and safety of immunotherapy for elderly patients in the "real-world".
The data of older NSCLC patients and younger patients who received immunotherapy between July 2018 to October 2021 were retrospectively analyzed and the objective response rate (ORR) and progression-free survival (PFS) in different age groups (less than 60 years old was defined as the young group, 60 years-74 years old was the young old group, 75 years old and above was the old old group) were compared. And the impact of different clinical characteristics on treatment response and prognosis were analyzed in each age subgroup.
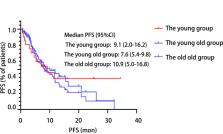
A total of 21 young patients, 70 young old patients and 15 old old patients were included in this study, with ORR of 33.3%, 52.8% and 53.3%, respectively, without statistically significant difference ( P=0.284). The median PFS was 9.1 mon, 7.6 mon and 10.9 mon, respectively, without statistically significant difference ( P=0.654). Further analysis of the predictors of immunotherapy in each subgroup revealed that patients in the young old group and young group who received immunotherapy in the first line had a longer PFS. The difference of the incidence of adverse events was not statistically significant among the three groups ( P>0.05).
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2022
- Record: found
- Abstract: found
- Article: not found
New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).
- Record: found
- Abstract: found
- Article: not found