- Record: found
- Abstract: found
- Article: found
Hydralazine loaded nanodroplets combined with ultrasound-targeted microbubble destruction to induce pyroptosis for tumor treatment
Read this article at
Abstract
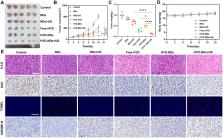
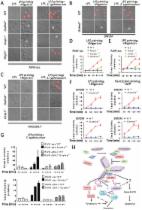
Pyroptosis, a novel type of programmed cell death (PCD), which provides a feasible therapeutic option for the treatment of tumors. However, due to the hypermethylation of the promoter, the critical protein Gasdermin E (GSDME) is lacking in the majority of cancer cells, which cannot start the pyroptosis process and leads to dissatisfactory therapeutic effects. Additionally, the quick clearance, systemic side effects, and low concentration at the tumor site of conventional pyroptosis reagents restrict their use in clinical cancer therapy. Here, we described a combination therapy that induces tumor cell pyroptosis via the use of ultrasound-targeted microbubble destruction (UTMD) in combination with DNA demethylation. The combined application of UTMD and hydralazine-loaded nanodroplets (HYD-NDs) can lead to the rapid release of HYD (a demethylation drug), which can cause the up-regulation of GSDME expression, and produce reactive oxygen species (ROS) by UTMD to cleave up-regulated GSDME, thereby inducing pyroptosis. HYD-NDs combined with ultrasound (US) group had the strongest tumor inhibition effect, and the tumor inhibition rate was 87.15% (HYD-NDs group: 51.41 ± 3.61%, NDs + US group: 32.73%±7.72%), indicating that the strategy had a more significant synergistic anti-tumor effect. In addition, as a new drug delivery carrier, HYD-NDs have great biosafety, tumor targeting, and ultrasound imaging performance. According to the results, the combined therapy reasonably regulated the process of tumor cell pyroptosis, which offered a new strategy for optimizing the therapy of GSDME-silenced solid tumors.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death.
- Record: found
- Abstract: not found
- Article: not found
Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a Gasdermin
- Record: found
- Abstract: found
- Article: found