- Record: found
- Abstract: found
- Article: found
Inhibitory Effect of β-Carotene on Helicobacter pylori-Induced TRAF Expression and Hyper-Proliferation in Gastric Epithelial Cells
Read this article at
Abstract
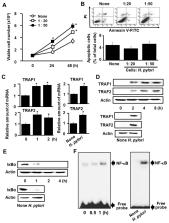
Helicobacter pylori infection causes the hyper-proliferation of gastric epithelial cells that leads to the development of gastric cancer. Overexpression of tumor necrosis factor receptor associated factor (TRAF) is shown in gastric cancer cells. The dietary antioxidant β-carotene has been shown to counter hyper-proliferation in H. pylori-infected gastric epithelial cells. The present study was carried out to examine the β-carotene mechanism of action. We first showed that H. pylori infection decreases cellular IκBα levels while increasing cell viability, NADPH oxidase activity, reactive oxygen species production, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, and TRAF1 and TRAF2 gene expression, as well as protein–protein interaction in gastric epithelial AGS cells. We then demonstrated that pretreatment of cells with β-carotene significantly attenuates these effects. Our findings support the proposal that β-carotene has anti-cancer activity by reducing NADPH oxidase-mediated production of ROS, NF-κB activation and NF-κB-regulated TRAF1 and TRAF2 gene expression, and hyper-proliferation in AGS cells. We suggest that the consumption of β-carotene-enriched foods could decrease the incidence of H. pylori-associated gastric disorders.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
- Record: found
- Abstract: found
- Article: not found
Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries.
- Record: found
- Abstract: found
- Article: not found