- Record: found
- Abstract: found
- Article: found
RhoA/Rho‐kinases in asthma: from pathogenesis to therapeutic targets
Read this article at
Abstract
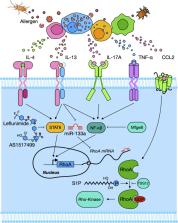
Asthma is a chronic and heterogeneous disease characterised by airway inflammation and intermittent airway narrowing. The key obstacle in the prevention and treatment of asthma has been our incomplete understanding of its aetiology and biological mechanisms. The ras homolog family member A (RhoA) of the Rho family GTPases has been considered to be one of the most promising and novel therapeutic targets for asthma. It is well known that RhoA/Rho‐kinases play an important role in the pathophysiology of asthma, including airway smooth muscle contraction, airway hyper‐responsiveness, β‐adrenergic desensitisation and airway remodelling. However, recent advances have suggested novel roles for RhoA in regulating allergic airway inflammation. Specifically, RhoA has been shown to regulate allergic airway inflammation through controlling Th2 or Th17 cell differentiation and to regulate airway remodelling through regulating mesenchymal stem cell (MSC) differentiation. In this review, we evaluate the literature regarding the recent advances in the activation of RhoA/Rho‐kinase, cytokine and epigenetic regulation of RhoA/Rho‐kinase, and the role of RhoA/Rho‐kinase in regulating major features of asthma, such as airway hyper‐responsiveness, remodelling and inflammation. We also discuss the importance of the newly identified role of RhoA/Rho‐kinase signalling in MSC differentiation and bronchial epithelial barrier dysfunction. These findings indicate the functional significance of the RhoA/Rho‐kinase pathway in the pathophysiology of asthma and suggest that RhoA/Rho‐kinase signalling may be a promising therapeutic target for the treatment of asthma.
Abstract
We evaluate recent advances in cytokine and epigenetic regulation of RhoA/Rho‐kinase and the role of RhoA/Rho‐kinase in regulating major clinical features of asthma, such as airway hyper‐responsiveness, remodelling and inflammation. The newly identified roles of RhoA/Rho‐kinase signalling in mesenchymal stem cell differentiation and bronchial epithelial barrier dysfunction are also discussed. We suggest that RhoA/Rho‐kinase signalling may be a promising therapeutic target for the treatment of asthma.
Related collections
Most cited references107
- Record: found
- Abstract: found
- Article: not found
T-helper type 2-driven inflammation defines major subphenotypes of asthma.
- Record: found
- Abstract: found
- Article: not found
Type 2 inflammation in asthma--present in most, absent in many.
- Record: found
- Abstract: found
- Article: not found
