- Record: found
- Abstract: found
- Article: found
Temporal Trends in the Use of Anticholinergic Drugs Among Older People Living in Long-Term Care Facilities in Helsinki
Read this article at
Abstract
Background
The use of drugs with anticholinergic properties (DAPs) is common among older adults despite their known adverse effects, such as cognitive decline. Professionals should pay attention to DAPs, since evidence on their adverse effects has been accumulating during the last decade. However, to our knowledge previous studies exploring temporal trends in the use of DAPs are scarce.
Objective
The aim of this study was to assess temporal trends in the use of DAPs from 2003 to 2017 in long-term care facilities in Helsinki.
Methods
Four cross-sectional studies were conducted in 2003, 2007, 2011, and 2017. Participants included older people (≥ 65 years) living in nursing homes (NHs) in 2003 ( n = 1979), 2011 ( n = 1568), and 2017 ( n = 750), and in assisted living facilities (ALFs) in 2007 ( n = 1336), 2011 ( n = 1556), and 2017 ( n = 1673) in Helsinki, Finland. Data on demographics, medication use, and diagnoses were collected by structured questionnaires. The assessments were conducted as a point prevalence over 1 day. The use of DAPs and the total anticholinergic burden were defined by the Anticholinergic Risk Scale (ARS).
Results
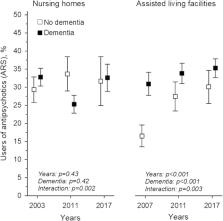
In ALFs, there has been an increasing trend in the use of DAPs over a 10-year period (41.2% in 2007 and 53.7% in 2017). In NHs, by contrast, the use of DAPs remained quite stable (52.3% in 2003 and 52.4% in 2017). The burden of DAPs measured by ARS score decreased in NHs and remained stable in ALFs. Marked changes occurred in the DAPs used; antidepressants, especially mirtazapine, increased in both settings, whereas the use of hydroxyzine and urinary antispasmodics nearly disappeared. The proportion of users of DAP antipsychotics increased in ALFs. Participants with dementia had a lower anticholinergic burden than those without dementia, in both settings.
Conclusions
Despite increased knowledge of the harms of DAPs, they remain widely used. Physicians seem to be aware of the harms of DAPs among people with dementia, and some other favorable trends in prescribing were also observed. Clinicians should especially consider the indications behind the use of DAP antidepressants and antipsychotics, and carefully weigh their potential benefits and harms.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
The anticholinergic risk scale and anticholinergic adverse effects in older persons.
- Record: found
- Abstract: found
- Article: not found
STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation.
- Record: found
- Abstract: found
- Article: not found