- Record: found
- Abstract: found
- Article: found
Apoptosis and non-alcoholic fatty liver diseases
Read this article at
Abstract
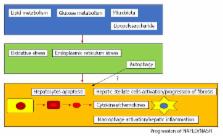
The number of patients with nonalcoholic fatty liver diseases (NAFLD) including nonalcoholic steatohepatitis (NASH), has been increasing. NASH causes cirrhosis and hepatocellular carcinoma (HCC) and is one of the most serious health problems in the world. The mechanism through which NASH progresses is still largely unknown. Activation of caspases, Bcl-2 family proteins, and c-Jun N-terminal kinase-induced hepatocyte apoptosis plays a role in the activation of NAFLD/NASH. Apoptotic hepatocytes stimulate immune cells and hepatic stellate cells toward the progression of fibrosis in the liver through the production of inflammasomes and cytokines. Abnormalities in glucose and lipid metabolism as well as microbiota accelerate these processes. The production of reactive oxygen species, oxidative stress, and endoplasmic reticulum stress is also involved. Cell death, including apoptosis, seems very important in the progression of NAFLD and NASH. Recently, inhibitors of apoptosis have been developed as drugs for the treatment of NASH and may prevent cirrhosis and HCC. Increased hepatocyte apoptosis may distinguish NASH from NAFLD, and the improvement of apoptosis could play a role in controlling the development of NASH. In this review, the association between apoptosis and NAFLD/NASH are discussed. This review could provide their knowledge, which plays a role in seeing the patients with NAFLD/NASH in daily clinical practice.
Related collections
Most cited references95
- Record: found
- Abstract: found
- Article: not found
The epidemiology of non-alcoholic fatty liver disease.
- Record: found
- Abstract: found
- Article: not found
Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice.
- Record: found
- Abstract: found
- Article: not found