- Record: found
- Abstract: found
- Article: found
PROCalcitonin-based algorithm for antibiotic use in Acute Pancreatitis (PROCAP): study protocol for a randomised controlled trial
Read this article at
Abstract
Background
Differentiating infection from inflammation in acute pancreatitis is difficult, leading to overuse of antibiotics. Procalcitonin (PCT) measurement is a means of distinguishing infection from inflammation as levels rise rapidly in response to a pro-inflammatory stimulus of bacterial origin and normally fall after successful treatment. Algorithms based on PCT measurement can differentiate bacterial sepsis from a systemic inflammatory response. The PROCalcitonin-based algorithm for antibiotic use in Acute Pancreatitis (PROCAP) trial tests the hypothesis that a PCT-based algorithm to guide initiation, continuation and discontinuation of antibiotics will lead to reduced antibiotic use in patients with acute pancreatitis and without an adverse effect on outcome.
Methods
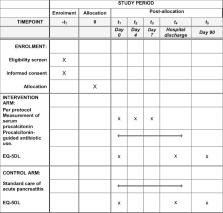
This is a single-centre, randomised, controlled, single-blind, two-arm pragmatic clinical and cost-effectiveness trial. Patients with a clinical diagnosis of acute pancreatitis will be allocated on a 1:1 basis to intervention or standard care. Intervention will involve the use of a PCT-based algorithm to guide antibiotic use. The primary outcome measure will be the binary outcome of antibiotic use during index admission. Secondary outcome measures include: safety non-inferiority endpoint all-cause mortality; days of antibiotic use; clinical infections; new isolates of multiresistant bacteria; duration of inpatient stay; episode-related mortality and cause; quality of life (EuroQol EQ-5D); and cost analysis. A 20% absolute change in antibiotic use would be a clinically important difference. A study with 80% power and 5% significance (two-sided) would require 97 patients in each arm (194 patients in total): the study will aim to recruit 200 patients. Analysis will follow intention-to-treat principles.
Discussion
When complete, PROCAP will be the largest randomised trial of the use of a PCT algorithm to guide initiation, continuation and cessation of antibiotics in acute pancreatitis. PROCAP is the only randomised trial to date to compare standard care of acute pancreatitis as defined by the International Association of Pancreatology/American Pancreatic Association guidelines to patients having standard care but with all antibiotic prescribing decisions based on PCT measurement.
Related collections
Most cited references20
- Record: found
- Abstract: not found
- Article: not found
Antibiotic-resistant bugs in the 21st century--a clinical super-challenge.
- Record: found
- Abstract: found
- Article: not found
Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms.
- Record: found
- Abstract: found
- Article: not found