- Record: found
- Abstract: found
- Article: found
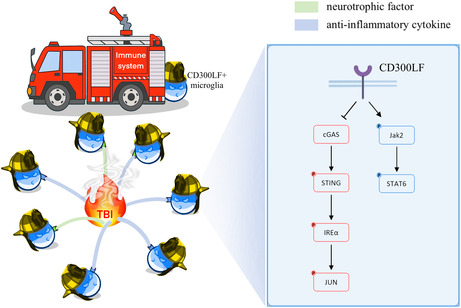
CD300LF + microglia impede the neuroinflammation following traumatic brain injury by inhibiting STING pathway

Read this article at
Abstract
Introduction
The diversity in microglial phenotypes and functions following traumatic brain injury (TBI) is poorly characterized. The aim of this study was to explore precise targets for improving the prognosis of TBI patients from a microglial perspective.
Results
In CD300LF‐deficient mice, we observed an increase in glial cell proliferation, more extensive neuronal loss, and worsened neurological function post‐TBI. Transcriptomic comparisons between CD300LF‐positive and CD300LF‐negative microglia illuminated that the neuroprotective role of CD300LF is principally mediated by the inhibition of the STING signaling pathway. In addition, this protective effect can be augmented using the STING pathway inhibitor C‐176.
Conclusions
Our research indicates that CD300LF reduces neuroinflammation and promotes neurological recovery after TBI, and that microglia are integral to the protective effects of CD300LF in this context. In summary, our findings highlight CD300LF as a critical molecular regulator modulating the adverse actions of microglia following acute brain injury and propose a novel therapeutic approach to enhance outcomes for patients with TBI.
Abstract
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Microglial and macrophage polarization—new prospects for brain repair.
- Record: found
- Abstract: found
- Article: not found
Microglia: active sensor and versatile effector cells in the normal and pathologic brain.
- Record: found
- Abstract: found
- Article: not found