- Record: found
- Abstract: found
- Article: found
Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals
Read this article at
Abstract
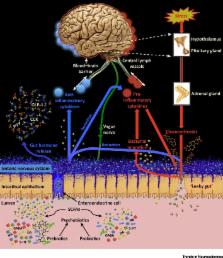
Psychobiotics were previously defined as live bacteria (probiotics) which, when ingested, confer mental health benefits through interactions with commensal gut bacteria. We expand this definition to encompass prebiotics, which enhance the growth of beneficial gut bacteria. We review probiotic and prebiotic effects on emotional, cognitive, systemic, and neural variables relevant to health and disease. We discuss gut–brain signalling mechanisms enabling psychobiotic effects, such as metabolite production. Overall, knowledge of how the microbiome responds to exogenous influence remains limited. We tabulate several important research questions and issues, exploration of which will generate both mechanistic insights and facilitate future psychobiotic development. We suggest the definition of psychobiotics be expanded beyond probiotics and prebiotics to include other means of influencing the microbiome.
Trends
Psychobiotics are beneficial bacteria (probiotics) or support for such bacteria (prebiotics) that influence bacteria–brain relationships.
Psychobiotics exert anxiolytic and antidepressant effects characterised by changes in emotional, cognitive, systemic, and neural indices. Bacteria–brain communication channels through which psychobiotics exert effects include the enteric nervous system and the immune system.
Current unknowns include dose-responses and long-term effects.
The definition of psychobiotics should be expanded to any exogenous influence whose effect on the brain is bacterially-mediated.
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression.
- Record: found
- Abstract: found
- Article: not found
Innate immune recognition of the microbiota promotes host-microbial symbiosis.
- Record: found
- Abstract: found
- Article: not found