- Record: found
- Abstract: found
- Article: found
Photochemical Formation and Electronic Structure of an Alkane σ-Complex from Time-Resolved Optical and X-ray Absorption Spectroscopy
Read this article at
Abstract

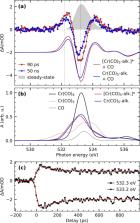
C–H bond activation reactions with transition metals typically proceed via the formation of alkane σ-complexes, where an alkane C–H σ-bond binds to the metal. Due to the weak nature of metal–alkane bonds, σ-complexes are challenging to characterize experimentally. Here, we establish the complete pathways of photochemical formation of the model σ-complex Cr(CO) 5-alkane from Cr(CO) 6 in octane solution and characterize the nature of its metal–ligand bonding interactions. Using femtosecond optical absorption spectroscopy, we find photoinduced CO dissociation from Cr(CO) 6 to occur within the 100 fs time resolution of the experiment. Rapid geminate recombination by a fraction of molecules is found to occur with a time constant of 150 fs. The formation of bare Cr(CO) 5 in its singlet ground state is followed by complexation of an octane molecule from solution with a time constant of 8.2 ps. Picosecond X-ray absorption spectroscopy at the Cr L-edge and O K-edge provides unique information on the electronic structure of the Cr(CO) 5-alkane σ-complex from both the metal and ligand perspectives. Based on clear experimental observables, we find substantial destabilization of the lowest unoccupied molecular orbital upon coordination of the C–H bond to the undercoordinated Cr center in the Cr(CO) 5-alkane σ-complex, and we define this as a general, orbital-based descriptor of the metal–alkane bond. Our study demonstrates the value of combining optical and X-ray spectroscopic methods as complementary tools to study the stability and reactivity of alkane σ-complexes in their role as the decisive intermediates in C–H bond activation reactions.
Related collections
Most cited references81
- Record: found
- Abstract: found
- Article: not found
Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy.
- Record: found
- Abstract: not found
- Article: not found
A new mixing of Hartree–Fock and local density-functional theories
- Record: found
- Abstract: not found
- Article: not found