- Record: found
- Abstract: found
- Article: found
Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents
Read this article at
Abstract
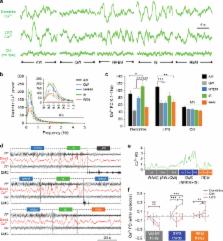
How sleep influences brain plasticity is not known. In particular, why certain electroencephalographic (EEG) rhythms are linked to memory consolidation is poorly understood. Calcium activity in dendrites is known to be necessary for structural plasticity changes, but this has never been carefully examined during sleep. Here, we report that calcium activity in populations of neocortical dendrites is increased and synchronised during oscillations in the spindle range in naturally sleeping rodents. Remarkably, the same relationship is not found in cell bodies of the same neurons and throughout the cortical column. Spindles during sleep have been suggested to be important for brain development and plasticity. Our results provide evidence for a physiological link of spindles in the cortex specific to dendrites, the main site of synaptic plasticity.
Abstract
Different stages of sleep, marked by particular electroencephalographic (EEG) signatures, have been linked to memory consolidation, but underlying mechanisms are poorly understood. Here, the authors show that dendritic calcium synchronisation correlates with spindle-rich sleep phases.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration.
- Record: found
- Abstract: found
- Article: not found
Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation.
- Record: found
- Abstract: found
- Article: not found