- Record: found
- Abstract: found
- Article: found
Assessment of 25-Year Survival of Women With Estrogen Receptor–Positive/ ERBB2-Negative Breast Cancer Treated With and Without Tamoxifen Therapy : A Secondary Analysis of Data From the Stockholm Tamoxifen Randomized Clinical Trial
Read this article at
Key Points
Question
Are clinically used markers of breast cancer, such as tumor size, tumor grade, progesterone receptor status, and Ki-67 status, independently associated with 25-year survival and tamoxifen treatment benefit among patients with breast cancer?
Findings
In this secondary analysis of data from 565 postmenopausal women with lymph node–negative, estrogen receptor–positive, and ERBB2-negative breast cancer who participated in the Stockholm tamoxifen randomized clinical trial (STO-3), tumor size and tumor grade were significantly associated with long-term (25-year) survival. A significant tamoxifen treatment benefit was observed among patients with larger tumors, lower tumor grades, and progesterone receptor–positive tumors.
Abstract
Importance
Clinically used breast cancer markers, such as tumor size, tumor grade, progesterone receptor (PR) status, and Ki-67 status, are known to be associated with short-term survival, but the association of these markers with long-term (25-year) survival is unclear.
Objective
To assess the association of clinically used breast cancer markers with long-term survival and treatment benefit among postmenopausal women with lymph node–negative, estrogen receptor [ER]–positive and ERBB2-negative breast cancer who received tamoxifen therapy.
Design, Setting, and Participants
This study was a secondary analysis of data from a subset of 565 women with ER-positive/ ERBB2-negative breast cancer who participated in the Stockholm tamoxifen (STO-3) randomized clinical trial. The STO-3 clinical trial was conducted from 1976 to 1990 and comprised 1780 postmenopausal women with lymph node–negative breast cancer who were randomized to receive adjuvant tamoxifen therapy or no endocrine therapy. Complete 25-year follow-up data through December 31, 2016, were obtained from Swedish national registers. Immunohistochemical markers were reannotated in 2014. Data were analyzed from April to December 2020.
Interventions
Patients in the original STO-3 clinical trial were randomized to receive 2 years of tamoxifen therapy vs no endocrine therapy. In 1983, patients who received tamoxifen therapy without cancer recurrence during the 2-year treatment and who consented to continued participation in the STO-3 study were further randomized to receive 3 additional years of tamoxifen therapy or no endocrine therapy.
Main Outcomes and Measures
Distant recurrence–free interval (DRFI) by clinically used breast cancer markers was assessed using Kaplan-Meier and multivariable Cox proportional hazards analyses adjusted for age, period of primary diagnosis, tumor size (T1a and T1b [T1a/b], T1c, and T2), tumor grade (1-3), PR status (positive vs negative), Ki-67 status (low vs medium to high), and STO-3 clinical trial arm (tamoxifen treatment vs no adjuvant treatment). A recursive partitioning analysis was performed to evaluate which markers were able to best estimate long-term DRFI.
Results
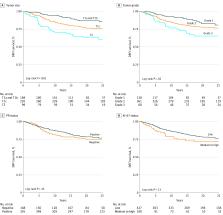
The study population comprised 565 postmenopausal women (mean [SD] age, 62.0 [5.3] years) with lymph node–negative, ER-positive/ ERBB2-negative breast cancer. A statistically significant difference in long-term DRFI was observed by tumor size (88% for T1a/b vs 76% for T1c vs 63% for T2 tumors; log-rank P < .001) and tumor grade (81% for grade 1 vs 77% for grade 2 vs 65% for grade 3 tumors; log-rank P = .02) but not by PR status or Ki-67 status. Patients with smaller tumors (hazard ratio [HR], 0.31 [95% CI, 0.17-0.55] for T1a/b tumors and 0.58 [95% CI, 0.38-0.88] for T1c tumors) and grade 1 tumors (HR, 0.48; 95% CI, 0.24-0.95) experienced a significant reduction in the long-term risk of distant recurrence compared with patients with larger (T2) tumors and grade 3 tumors, respectively. A significant tamoxifen treatment benefit was observed among patients with larger tumors (HR, 0.53 [95% CI, 0.32-0.89] for T1c tumors and 0.34 [95% CI, 0.16-0.73] for T2 tumors), lower tumor grades (HR, 0.24 [95% CI, 0.07-0.82] for grade 1 tumors and 0.50 [95% CI, 0.31-0.80] for grade 2 tumors), and PR-positive status (HR, 0.38; 95% CI, 0.24-0.62). The recursive partitioning analysis revealed that tumor size was the most important characteristic associated with long-term survival, followed by clinical trial arm among patients with larger tumors.
Conclusions and Relevance
This secondary analysis of data from the STO-3 clinical trial indicated that, among the selected subgroup of patients, tumor size followed by tumor grade were the markers most significantly associated with long-term survival. Furthermore, a significant long-term tamoxifen treatment benefit was observed among patients with larger tumors, lower tumor grades, and PR-positive tumors.
Abstract
This secondary analysis of the Stockholm tamoxifen clinical trial examines the association of clinical breast cancer markers with 25-year survival and tamoxifen treatment benefit among postmenopausal women with lymph node–negative ER-positive/ ERBB2-negative breast cancer.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years.
- Record: found
- Abstract: found
- Article: found
Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials
- Record: found
- Abstract: found
- Article: not found