- Record: found
- Abstract: found
- Article: found
Toxicity of advanced glycation end products (Review)

Read this article at
Abstract
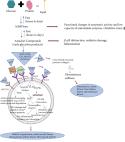
Advanced glycation end-products (AGEs) are proteins or lipids glycated nonenzymatically by glucose, or other reducing sugars and their derivatives, such as glyceraldehyde, glycolaldehyde, methyloglyoxal and acetaldehyde. There are three different means of AGE formation: i) Maillard reactions, the polyol pathway and lipid peroxidation. AGEs participate in the pathological mechanisms underlying the development of several diseases, such as diabetes and its complications, retinopathy or neuropathy, neurological disorders (for example, Parkinson's disease and Alzheimer's disease), atherosclerosis, hypertension and several types of cancer. AGE levels are increased in patients with hyperglycaemia, and is likely the result of the high concentration of glycation substrates circulating in the blood. The present review summarises the formation and nomenclature of advanced glycation end-products, with an emphasis on the role of AGEs in the development of diabetes, neurological disorders, as well as in cancer and other pathologies. A particular focus is placed on the functions of toxic AGEs. Additionally, studies which have shown the cytotoxicity of glycated albumin and other AGEs are also discussed. Finally, the diagnostic relevance of AGEs as well as for targeting in therapeutic strategies are highlighted.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications and other age-related diseases

- Record: found
- Abstract: found
- Article: found
Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases
- Record: found
- Abstract: found
- Article: found