- Record: found
- Abstract: found
- Article: found
Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer
Read this article at
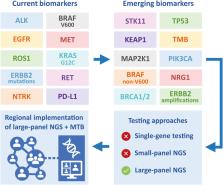
Summary
For patients with advanced stage non-small cell lung cancer (NSCLC), treatment strategies have changed significantly due to the introduction of targeted therapies and immunotherapy. In the last few years, we have seen an explosive growth of newly introduced targeted therapies in oncology and this development is expected to continue in the future. Besides primary targetable aberrations, emerging diagnostic biomarkers also include relevant co-occurring mutations and resistance mechanisms involved in disease progression, that have impact on optimal treatment management. To accommodate testing of pending biomarkers, it is necessary to establish routine large-panel next-generation sequencing (NGS) for all patients with advanced stage NSCLC. For cost-effectiveness and accessibility, it is recommended to implement predictive molecular testing using large-panel NGS in a dedicated, centralized expert laboratory within a regional oncology network. The central molecular testing center should host a regional Molecular Tumor Board and function as a hub for interpretation of rare and complex testing results and clinical decision-making.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology.
- Record: found
- Abstract: found
- Article: not found
Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group
- Record: found
- Abstract: found
- Article: not found