- Record: found
- Abstract: found
- Article: found
The cGAS-STING pathway: The role of self-DNA sensing in inflammatory lung disease
Read this article at
Abstract
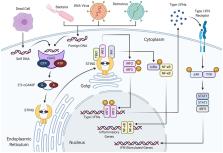
The presence of DNA in the cytosol is usually a sign of microbial infections, which alerts the host innate immune system to mount a defense response. Cyclic GMP-AMP synthase (cGAS) is a critical cytosolic DNA sensor that elicits robust innate immune responses through the production of the second messenger, cyclic GMP-AMP (cGAMP), which binds and activates stimulator of interferon genes (STING). However, cGAS binds to DNA irrespective of DNA sequence, therefore, self-DNA leaked from the nucleus or mitochondria can also serve as a cGAS ligand to activate this pathway and trigger extensive inflammatory responses. Dysregulation of the cGAS-STING pathway is responsible for a broad array of inflammatory and autoimmune diseases. Recently, evidence has shown that self-DNA release and cGAS-STING pathway over-activation can drive lung disease, making this pathway a promising therapeutic target for inflammatory lung disease. Here, we review recent advances on the cGAS-STING pathway governing self-DNA sensing, highlighting its role in pulmonary disease.
Related collections
Most cited references162

- Record: found
- Abstract: found
- Article: found
A new coronavirus associated with human respiratory disease in China
- Record: found
- Abstract: found
- Article: not found
Pattern recognition receptors and inflammation.
- Record: found
- Abstract: found
- Article: not found