- Record: found
- Abstract: found
- Article: found
Improving CBCT image quality to the CT level using RegGAN in esophageal cancer adaptive radiotherapy
Read this article at
Abstract
Objective
This study aimed to improve the image quality and CT Hounsfield unit accuracy of daily cone-beam computed tomography (CBCT) using registration generative adversarial networks (RegGAN) and apply synthetic CT (sCT) images to dose calculations in radiotherapy.
Methods
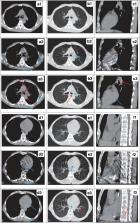
The CBCT/planning CT images of 150 esophageal cancer patients undergoing radiotherapy were used for training (120 patients) and testing (30 patients). An unsupervised deep-learning method, the 2.5D RegGAN model with an adaptively trained registration network, was proposed, through which sCT images were generated. The quality of deep-learning-generated sCT images was quantitatively compared to the reference deformed CT (dCT) image using mean absolute error (MAE), root mean square error (RMSE) of Hounsfield units (HU), and peak signal-to-noise ratio (PSNR). The dose calculation accuracy was further evaluated for esophageal cancer radiotherapy plans, and the same plans were calculated on dCT, CBCT, and sCT images.
Results
The quality of sCT images produced by RegGAN was significantly improved compared to the original CBCT images. ReGAN achieved image quality in the testing patients with MAE sCT vs. CBCT: 43.7 ± 4.8 vs. 80.1 ± 9.1; RMSE sCT vs. CBCT: 67.2 ± 12.4 vs. 124.2 ± 21.8; and PSNR sCT vs. CBCT: 27.9 ± 5.6 vs. 21.3 ± 4.2. The sCT images generated by the RegGAN model showed superior accuracy on dose calculation, with higher gamma passing rates (93.3 ± 4.4, 90.4 ± 5.2, and 84.3 ± 6.6) compared to original CBCT images (89.6 ± 5.7, 85.7 ± 6.9, and 72.5 ± 12.5) under the criteria of 3 mm/3%, 2 mm/2%, and 1 mm/1%, respectively.
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Conference Proceedings: not found
Image-to-Image Translation with Conditional Adversarial Networks
- Record: found
- Abstract: not found
- Conference Proceedings: not found
Unpaired Image-to-Image Translation Using Cycle-Consistent Adversarial Networks
- Record: found
- Abstract: found
- Article: not found