- Record: found
- Abstract: found
- Article: found
Schizophrenia Genomics: Convergence on Synaptic Development, Adult Synaptic Plasticity, or Both?
Read this article at
Abstract
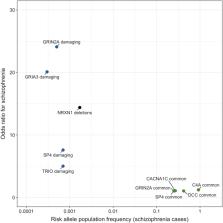
Large-scale genomic studies of schizophrenia have identified hundreds of genetic loci conferring risk to the disorder. This progress offers an important route toward defining the biological basis of the condition and potentially developing new treatments. In this review, we discuss insights from recent genome-wide association study, copy number variant, and exome sequencing analyses of schizophrenia, together with functional genomics data from the pre- and postnatal brain, in relation to synaptic development and function. These data provide strong support for the view that synaptic dysfunction within glutamatergic and GABAergic (gamma-aminobutyric acidergic) neurons of the cerebral cortex, hippocampus, and other limbic structures is a central component of schizophrenia pathophysiology. Implicated genes and functional genomic data suggest that disturbances in synaptic connectivity associated with susceptibility to schizophrenia begin in utero but continue throughout development, with some alleles conferring risk to the disorder through direct effects on synaptic function in adulthood. This model implies that novel interventions for schizophrenia could include broad preventive approaches aimed at enhancing synaptic health during development as well as more targeted treatments aimed at correcting synaptic function in affected adults.
Related collections
Most cited references128
- Record: found
- Abstract: found
- Article: found
Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression
- Record: found
- Abstract: found
- Article: not found
Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism
- Record: found
- Abstract: found
- Article: not found