- Record: found
- Abstract: found
- Article: found
Atlastins remodel the endoplasmic reticulum for selective autophagy
Read this article at
Abstract
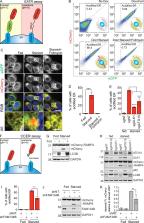
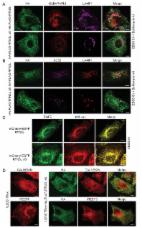
Multiple ER-phagy receptors have been reported recently, but other mediators or regulators have remained elusive. Liang et al. developed two ER-phagy–specific reporter assays and showed that Atlastins, a family of ER surface GTPases, are positive regulators of ER-phagy that act downstream of an ER-phagy receptor, FAM134B.
Abstract
Specific receptors are required for the autophagic degradation of endoplasmic reticulum (ER), known as ER-phagy. However, little is known about how the ER is remodeled and separated for packaging into autophagosomes. We developed two ER-phagy–specific reporter systems and found that Atlastins are key positive effectors and also targets of ER-phagy. Atlastins are ER-resident GTPases involved in ER membrane morphology, and Atlastin-depleted cells have decreased ER-phagy under starvation conditions. Atlastin’s role in ER-phagy requires a functional GTPase domain and proper ER localization, both of which are also involved in ER architecture. The three Atlastin family members functionally compensate for one another during ER-phagy and may form heteromeric complexes with one another. We further find that Atlastins act downstream of the FAM134B ER-phagy receptor, such that depletion of Atlastins represses ER-autophagy induced by the overexpression of FAM134B. We propose that during ER-phagy, Atlastins remodel ER membrane to separate pieces of FAM134B-marked ER for efficient autophagosomal engulfment.
Related collections
Most cited references20
- Record: found
- Abstract: found
- Article: not found
A class of membrane proteins shaping the tubular endoplasmic reticulum.
- Record: found
- Abstract: found
- Article: found
Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy
- Record: found
- Abstract: found
- Article: not found
Cleaning up: ER-associated degradation to the rescue.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.